
Главная страница Случайная страница
Разделы сайта
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Inquiry 4 страница
|
|
This includes bronchoscopy and thoracoscopy. Bronchoscopy is used to inspect the tracheal and bronchial mucosa of the second and third order. It is performed by means of a special apparatus known as a bron-chofibroscope (Fig. 20). Special forceps are used with a bronchoscope to take samples for biopsy, extract foreign bodies, or polyps. A photographic device is also used with the bronchoscope.
Mucosa of the upper airway is first anaesthetized by a 1-3 per cent di-caine solution. Next a bronchoscope is introduced via the mouth into the vocal slit and farther into the trachea. The physician examines the mucosa
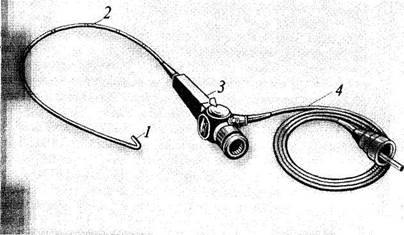
Fig. 20. Bronchofibroscope.
/—controllable distal end; 2 —flexible rod; 3 —instrument body with an eye-piece and control handle.
4 —fibre-optic cable.
f the bronchi and the trachea. Using special forceps, tissue samples can be ken from a suspected area (biopsy) for histological and cytological analysis (Plate 5). Photography can also be made whenever necessary.
Bronchoscopy is used for diagnosing erosions and ulcers of the bronchial mucosa and tumours of the bronchial wall, removing foreign bodies, extracting polyps, and treating bronchiectasis and centrally located abscesses of the lungs. Sputum containing pus is first aspirated through the bronchoscope, and then antibiotics are administered into the bronchial lumen or cavity.
Thoracoscopy is carried out with a special electrically lighted instrument known as a thoracoscope that consists of a hollow metal tube and a special optic device. Thoracoscopy is used for examination of the visceral and parietal pleura and for severance of pleural adhesion bands that may interfere with placing artificial pneumothorax (in cavernous pulmonary tuberculosis).
METHODS FOR FUNCTIONAL DIAGNOSIS
Functional studies of the external respiratory system are very important for an integrated examination of patients with diseases of the lungs and bronchi. A functional study cannot diagnose the disease which caused the respiratory insufficiency, but often reveals it long before its clinical symptoms are apparent, establishes its type, character and degree, and can be used to follow up functional changes in the external respiratory apparatus in the course of the disease and under the effect of treatment.
Lung ventilation. The indices of lung ventilation are not constant and depend not only on the pathological conditions of the lungs or bronchi, but also on the patient's constitution, physical fitness, height, weight, sex, and age. The data obtained during examination of the patient are therefore assessed by comparing them with the data that might be expected from a person with the given physical properties. These data are calculated by special nomograms and formulas that have been compiled from basal metabolism indices.
Measuring respiratory capacity. Various indices are used to characterize lung ventilation. The so-called volumes of the lungs are most Popular but they are not accurate enough.
1. The respiratory volume (RV) is the volume of air inspired and ex-Pired during normal breathing. It is 500 ml on the average varying from 300 to 900 ml. Of this volume, about 150 ml is the physiological dead-space volume of air (PDSV) which is present in the larynx, trachea, and ronchi, but which does not participate in respiratory exchange. It should however be remembered that the air of the PDSV is mixed with the inspired
1O-15S6
Special Part
r
Chapter 5. Respiratory System
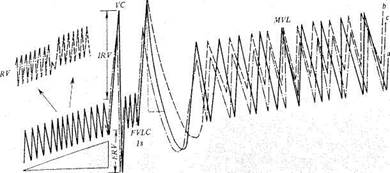
|
| FVLC |
| RV |
air to warm and moisten it, which makes residual air physiologically ijjj. portant.
2. The expiratory reserve volume (ERV) (1500-2000 ml). This is the
volume of air which can be expired by maximum effort after completion of
a normal expiration.
3. The inspiratory reserve volume (IRV) (1500-2000 ml). This is the
volume of air that can be inspired after a normal inspiration.
4. The vital capacity (VC) is found by summation of the IRV and ERV
and the respiratory volume (3700 ml on the average). This is the greatest
volume of air that can be expired from the lungs after a maximum inspira
tion. The vital capacity of the lungs can be calculated by multiplying the
tabulated (optimal) volume of basal metabolism by an empirically found
factor 2.3. The deviation from the expected (optimum) vital capacity
calculated by this method should not exceed ± 15 per cent.
5. The residual air volume (RAV) (1000-1500 ml) is the air that re
mains in the lungs after maximum expiration.
6. The total lung capacity (TLC) is the sum of the RV, ERV and IRV,
and RAV. It is about 5000-6000 ml.
Respiratory volumes can be used to assess possible compensation of respiratory insufficiency by increasing respiratory depth at the expense of expiration and inhalation and residual volume.
Normal respiratory volume is about 15 per cent of the vital lung capacity; expiratory and inspiratory air volumes are 42—43 per cent (inspiratory air usually slightly exceeds expiratory air volume); residual air is about 33 per cent of the vital capacity of the lungs. The VC slightly decreases in patients with obstructive hypoventilation, while expiratory and residual air volumes increase at the expense of decreased inspiratory air. RAV (especially the RAV: TLC ratio) increases in some cases to 50 per cent of the TLC (in lung emphysema, bronchial asthma, to a lesser degree in aged persons). VC in patients with hypoventilation also decreases because of the decreased IRV, while the RAV changes only insignificantly.
Spirography gives more reliable information on respiratory volumes (Fig. 21). A spirograph can be used not only to measure various respiratory volumes but also some additional ventilation characteristics such as the respiratory volume, minute volume, maximum ventilation of the lungs, and the volume of forced expiration. A spirograph can also be used with a bronchoscope to determine all indices separately for each lung (bron-chospirography). Using an absorber of carbon dioxide, it is possible to determine oxygen absorption per minute.
The RAV can also be determined by spirography. A spirograph with a closed system and a carbon dioxide absorber is used for the purpose. The apparatus is filled with pure oxygen and the patient breathes into the ap-
Fig. 21. Spirograms of a healthy individual (a) and of patients with obstructive (b) and restrictive (c) respiratory insufficiency.
paratus for ten minutes, after which the RAV is determined by calculating the amount and concentration of nitrogen captured by the spirograph from
the patient's lungs.
It is difficult to determine the PDSV. It can only be assessed by calculating the ratio of partial pressure of CO2 in the expired air and the arterial blood. PDSV increases in patients with large caverns and in the presence of ventilated pulmonary areas that are not sufficiently supplied with blood.
Intensity of lung ventilation. 1. The minute volume (MV) is calculated by multiplying the respiratory volume by respiratory rate; it is about 5000 ml on the average. More accurately the MV can be determined by a Douglas bag or using a spirograph.
2. The maximum lung ventilation (MLV) is the amount of air that can
be handled by the lungs by maximum effort of the respiratory system. It is
determined by spirometry during deepest breathing at a rate of 50 r/min;
normal ventilation is 80-200 1/min. According to Dembo, the predicted
value of the maximum ventilation is the vital capacity of the lungs
multiplied by 35 (MLV = VC x 35).
3. The respiratory reserve (RR) is determined by the formula RR =
= MLV - MV. In norm the RR exceeds the MV by at least 15-20 times.
In healthy persons the RR is 85 per cent of the MLV, while in patients with
Special Part
Chapter 5. Respiratory System
respiratory insufficiency it decreases to 60 per cent or lower. This value shows the reserves of a healthy person by which he ensures adequate ventilation under considerable loads, or of a patient with respiratory insufficiency by which he may compensate for significant insufficiency by increasing the minute respiratory volume.
All these tests help study lung ventilation and its reserves, which are important when heavy work is done or there are respiratory diseases.
Mechanics of the respiratory act. The study of this mechanics is necessary for determining changes in the inspiration to expiration ratio, the respiratory effors at various respiratory phases, and other indices.
1. The forced expiratory vital capacity (FEVC). According to Votchal-Tiffeneau this is determined like the vital capacity except that the forced expiration should be performed as fast as possible. The FEVC is 8—11 per cent (100-300 ml) lower than the VC in healthy persons, mainly due to the increased resistance of fine bronchi to the passage of air. When this resistance increases due to bronchitis, bronchospasm, emphysema, etc., the difference may be as great as 1500 ml and more. The volume of forced expiration per minute is also determined. In healthy persons it is 82.7 per cent of the VC. The length of the forced expiration until the moment it slows abruptly is also determined. These investigations can only be done with a spirograph. Broncholytics (e.g. using theophedrine) to determine the FEVC or the various modifications of this test enable us to assess the role of bronchospasm in the aetiology of the respiratory insufficiency and decreased values of the above indices. If, after giving theophedrine, the findings remain markedly subnormal bronchospasm is not the cause of their reduction.
2. The forced inspiratory vital capacity (FIVC) is determined during
forced inspiration at a maximum speed. It does not change in emphysema
non-aggravated by bronchitis but decreases in obstructed patency of the
airways.
3. Pneumotachymetry is the technique used for measuring peak
velocities of air streams in forced inspiration and expiration and is intended
to determine the condition of bronchial patency.
4. Pneumotachygraphy is measuring the volumetric rate and pressure
arising at various phases of respiration (both normal and forced). The in
strument used for the purpose is known as a pneumotachygraph. The
technique is based on recording pressures of air streams at various points
and during different phases of the respiratory cycle. Pneumotachygraphy
is used to determine the volumetric rate of air streams during both inspira
tion and expiration (in normal breathing it is about 300—500 ml/s, and in
forced respiration 5000-8000 ml/s), the length of the inspiration and ex
piration phases, the minute volume, intra-alveolar pressure, resistance of
the air ducts to the air stream, distensibility of the lungs and the chest wall, the mechanism of respiratory movements, and some other indices.
Tests for apparent and latent respiratory insufficiency. Oxygen consumption and oxygen deficit are determined by spirography with a closed CO2 absorption system. In determining oxygen deficit, a spirogram is compared with another spirogram obtained in the same conditions but with the apparatus filled with oxygen.
Ergospirography is the method by which the amount of work that a patient can perform without showing signs of respiratory insufficiency is determined. The method is thus suitable for the study of the respiratory reserves of man. It is also used for determining oxygen consumption and oxygen deficit in resting patients and during exercise on an ergometer. Respiratory insufficiency is assessed by the presence of spirographic oxygen deficit of more than 100 1/min or latent oxygen deficit of more than 20 per cent (respiration becomes more quiet when oxygen breathing is substituted for normal air breathing), and also by the change in partial pressure of oxygen and carbon dioxide of the blood.
Study of blood gases. Blood samples are obtained from the skin of a warmed up finger which is pierced through with a needle. It has been proved that the gas composition of the blood taken from the finger tip is similar to that of the arterial blood. The blood sample is transferred into a measuring cylinder where it is kept under a layer of warm vaseline oil (to prevent oxidation by atmospheric oxygen). A Van-Slike apparatus is used for the study of the gas composition of blood. The technique is based on displacement of gases from their combination with haemoglobin into vacuum (by chemical means). The following is determined: (1) oxygen content, in units of volume; (2) oxygen capacity of blood (i.e. the amount of oxygen that can be bound by a given blood unit); (3) percentage of oxygen saturation of the blood (normal, 95); (4) partial pressure of oxygen (normal, 90—100 mm Hg); (5) carbon dioxide content in arterial blood (normal, about 48% v/v); (6) partial pressure of carbon dioxide (normal, about 40 mm Hg). Partial tension of gases (O2 and COj) in arterial blood can now be determined by a special apparatus, or by some other techniques.
The oxygen saturation of the blood can also be determined by ox-yhaemometry. A photoelectric device is attached to the patient's ear lobe and the scale of the instrument reads oxygen saturation during breathing with air and oxygen. A great difference in the readings indicates oxygen deficit in the blood.
Determining separately the flow rate in the greater and the lesser cir-culation gives important diagnostic and prognostic information in patients Wltn dysfunction of the external respiration.
150 Special Part
PLEUROCENTESIS
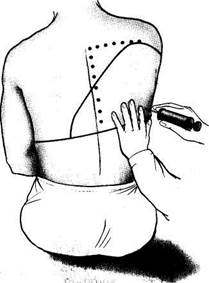
|
| Fig. 22. Pleurocentesis. |
Pleurocentesis is used: (1) to take samples of the pleural fluid for diagnostic studies, (2) to remove fluids from the pleural cavity, and, whenever necessary, to administer medicinal preparations. During the operation the patient sits on a chair, facing the chair back, with his arms crossed on the chest. The site of puncture is treated with an alcohol iodine solution and then with an anaesthetic. The chest is punctured in the posterior axillary line at the point of maximum dullness, which is preliminarily determined by percussion (this is usually the 7th or 8th intercostal space); the puncture is made at the upper edge of the underlying rib, because intercostal vessels are invested at the lower margin of the rib (Fig. 22). An exploratory puncture is done by a syringe (10 ml) with a thick and long needle. Large amounts of fluids are aspired from the pleura by the Patain apparatus or by an electric pump. As the needle enters the pleural cavity the physician " feels" a free space. The needle sometimes meets resistance which is usually thickened pleura. The amount of fluid taken for diagnostic purposes is 50—150 ml. The fluid undergoes physicochemical, cytological, and bacteriological analyses. If much fluid is present in the pleural cavity, 800—1200 ml is first taken. If larger amounts of the liquid are removed, the mediastinal organs may be quickly shifted toward the affected side and collapse may occur. After the needle has been removed, the punctured site should be treated with a 5 per cent iodine solution.
Chapter 5. Respiratory System 131
I LABORATORY STUDIES
Study of the sputum. Sputum is a pathological material that is expelled from the respiratory organs during the coughing act. Sputum may contain mucoid secretions, serous fluid, blood cells, desquamated respiratory epithelium, protozoa, and, in rare cases, helminths and their ova. The study of the sputum gives information concerning the pathology of the respiratory organs and in some cases helps establish its aetiology.
The morning sputum taken before breakfast (after mouth rinsing) is the best material for examination. If the sputum is scarce, it should be collected during one or two days for examination for the presence of tuberculosis mycobacteria. Saprophytic flora rapidly multiplies in sputum to destroy the formed blood elements. Special calibrated bottles provided with screw caps should be used for gathering sputum.
The study begins with observation of the sputum first in a transparent bottle and then in a Petri dish which is placed alternately on the black and white surface. General properties, colour, and consistency of the sputum are noted. Mucoid sputum is usually colourless; it occurs in acute bronchitis. Serous sputum is also colourless, liquid, and foamy; it occurs in pulmonary oedema. Mucopurulent sputum is yellow or greenish and tenacious; it is characteristic of chronic bronchitis, tuberculosis, etc. Purulent uniform semiliquid sputum with a greenish-yellow tint is typical of the ruptured lung abscess. Sputum may contain blood. It occurs in pulmonary haemorrhage (tuberculosis, cancer, bronchiectasis). Sputum may also be mucopurulent with streaks of blood (in bronchiectasis), serous blood-stained foamy (in lung oedema), mucous bloody (in lung infarction or in congestion in the lesser circulation), or bloody purulent (in gangrene and abscess of the lung). If blood is not expectorated from the respiratory tract immediately but stays there for some time, the haemoglobin converts into haemosiderin to give a rusty hue to the sputum, which is characteristic of acute lobar pneumonia.
Sputum may form layers on standing. Three-layer sputum is characteristic of chronic purulent processes. The upper layer is mu'copurulent, the middle one serous, and the lower layer is pus. Purulent sputum is sometimes separated into two layers, i.e. serous and purulent. Sputum is usually odourless. Foul odour of freshly expectorated sputum depends on the putrefactive decompositon of tissues (gangrene, degrading cancer tumour) or on the decomposed protein of the sputum reined in various cavities (abscess, bronchiectases).
The following elements can be seen in the sputum by an unaided eye: urschmann spirals (in the form of small dense twisted threads); fibrin °ts (whitish and reddish branching elastic formations, occurring in
Special Part
Chapter 5. Respiratory System
fibrinous bronchitis and sometimes in pneumonia); compact lenticular greenish-yellow formations consisting of calcified elastic fibres, cholesterol crystals and soaps containing tuberculosis mycobacteria; Dittrich 's plugs that resemble the lenticular formations in appearance and composition but free of tuberculosis mycobacteria and having offensive odour on pressing (occur in gangrene, chronic abscess, and fetid bronchitis); lime grains, that are found during decompositon of old tuberculosis foci; actinomycete drusen in the form of yellow formations resembling coarse flour; necrotiz-ed pieces of lung tissues and tumours; food remains.
The medium of the sputum is alkaline as a rule; it becomes acid in the presence of gastric juice and during decomposition; this helps differentiate between haemoptysis and haematemesis.
Microscopic study of the sputum can be done with native and stained preparations. In the first case, small clots or white threads of the purulent and blood stained material are taken from a sputum sample and transferred onto an object to make a thin semitransparent preparation which is covered by another glass. Examination begins with general observation at a small magnification in order to identify Curschmann spirals. Formed elements are then differentiated at a greater magnification. Curschmann spirals are mucous threads consisting of a dense central filament and a spiral mantle containing leucocytes (often eosinophils) and Charcot-Leyden crystals (Fig. 23). Curschmann spirals are found in the sputum during bronchial spasms, mostly in bronchial asthma, less frequently in pneumonia and lung cancer.
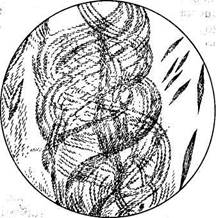
|
| Fig. 23. Curschmann's spirals and Charcot-Leyden crystals in sputum. |
Leucocytes can be found in native preparations at large magnification. A small quantity of leucocytes can be found in any sputum, while their
large amounts are characteristic of inflammatory and especially purulent processes. Eosinophils (Plate 6) can be identified in the native preparation by their uniform large lustrous grains, but they are better identified by staining. Erythrocytes (red blood cells) appear during decomposition of lung tissue, in pneumonia, congestion in the lesser circulation, lung infarction, etc.
Squamous epithelium gets into the sputum mostly from the mouth and is diagnostically unimportant. Columnar ciliated epithelium is contained in small quantity in any sputum, but its large amounts are found in bronchitis, bronchial asthma, and other affections of the respiratory ducts. Alveolar macrophages are large cells (twice or thrice as great as leucocytes) of reticulohistiocyte aetiology. Their cytoplasm contains many inclusions. They can be colourless (meylin grains), black from coal particles (dust cells) (Plate 7), or brown-yellow from haemosiderin (heart-disease cells or siderophages). Small quantities of alveolar macrophages are contained in any sputum but their large amounts are found in inflammatory diseases. Heart-disease cells (Plate 8) occur where erythrocytes get into the alveolar cavities (in congestion of the lesser circulation, especially in mitral stenosis, lung infarction, and also in acute lobar pneumonia). Berlin blue is used for their reliable identification. A small quantity of sputum is placed on an object glass, 1—2 drops of a 5 per cent potassium ferrocyanide solution are added, and the same amount of a 2 per cent hydrochloric acid added in 2-3 minutes; the material is mixed and is covered with another glass. Haemosiderin grains become stained blue in a few minutes.
Malignant tumour cells are often present in the sputum, especially so if the tumour degrades or grows endobronchially. These cells can easily be identified by their atypical view: they are mostly large and disfigured, their nuclei are large; several nuclei are sometimes found in one cell. Lining epithelium of the bronchi becomes metaplastic in chronic inflammation; it acquires atypical features and may resemble tumour cells. Cells can therefore be identified as tumour cells only in the presence of the whole complex of atypical and polymorphous cells, especially if they are found on fibres or together with elastic fibres.
Elastic fibres (Plate 9) are found in the sputum during decomposition °f the lung tissue in tuberculosis, cancer and abscess. Elastic fibres are fine formations of two dichotomically branching filaments of the uniform thickness. They often occur as coils in alveoli. Since these fibres do not occur in every drop of the sputum, they should be concentrated to make their search easier. To that end, a few millilitres of sputum are mixed with an eQual, (or double) quantity of a 10 per cent potassium hydroxide solution and heated to dissolve the mucus. All formed elements of the sputum, ex-CePt elastic fibres, are dissolved; the mixture is then cooled, centrifuged,
Special Part
Chapter 5. Respiratory System.
3-5 drops of a 1 per cent alcohol eosin solution are added, and the precipitate examined in a microscope. Elastic fibres preserve their character and are clearly seen as bright red formations.
Actinomycetes are separated from small yellow compact grains (drusen) of sputum. When a druse is compressed in a drop of glycerol between two object glasses, its central part consisting of interlaced mycelium and radiant flask-shaped formations can be seen in a microscope. When the crushed druse is stained after Gram, the mycelium acquires violet and the flask crimson colouration. Another important fungus occurring in the sputum is Candida albicans, which affects the lungs during prolonged antibiotic therapy of asthenic patients. Budding yeast-like cells and branching mycelium (on which the spores are arranged in churns) are found in the native preparation.
Sputum can contain Charcot-Leyden crystals. These are colourless oc-tahedra of various size, resembling the pointer of a compass. They consist of protein released during decomposition of eosinophils and are therefore found in the sputum containing much eosinophils. Old sputum contains greater amount of these crystals. Haematoidin can be found in the sputum after pulmonary haemorrhage (provided blood is not liberated with the sputum immediately). Crystals of haematoidin are rhombic or needle-shaped brown-yellow formations.
Microscopy of stained preparations is carried out to study microbial flora of the sputum and of some of its cells. Determination of malignant tumour cells is the most important object of microscopy. A smear of suspected material found in the native preparation is carefully fixed (not to crush cells) in methyl alcohol or Nikiforov mixture and stained after Romanovsky-Giemsa (or by some other differentiating staining). Tumour cells are characterized by polymorphism and variable size, the presence of very large cells, large (often hyperchromic and also hypochromic) nuclei, sometimes multiple irregular nuclei with large nucleoli; homogeneous, sometimes vacuolized cytoplasm, markedly basophilic in some cells; and also mytotic figures (in some cases). Complexes of polymorphic cells of the described character are more important diagnostically.
Romanovsky-Giemsa stained smears are suitable for identification of eosinophilic leucocytes. Another staining is also useful: the material is first treated with a 1 per cent eosin solution (2-3 minutes) and then with a 0.2 per cent methylene blue solution (0.5-1 minute). Single eosinophils can occur in any sputum; large amounts of eosinophils (up to 50—90 per cent of all leucocytes) are found in bronchial asthma, eosinophilic infiltrations, helminthic invasions of the lungs, etc.
Smears for bacterioscopic studies are prepared by crushing a clot of sputum between two object glasses. The dried smear is fixed by passing it
three times through a flame of a gas burner, and then stained; Ziehl-Neelsen staining is used to detect tuberculosis mycobacteria, and Gram staining in other cases.
Ziehl-Neelsen staining. A piece of blotting paper, the size of the smear, is placed over a smear fixed on a glass, Ziehl carbol fuchsine solution is then used to wet the paper and the preparation is held in a low flame of a burner until vapour appears. The paper is then removed, the preparation is washed with water and decoloured in a 3 per cent hydrochloric acid solution and 96 per cent alcohol (or in a 5—10 per cent sulphuric acid solution). The preparation is then washed in water thoroughly, and stained again for 0.5— 1 minute in a 0.5 per cent methylene blue solution. A final washing is then given. Acid-fast bacteria are thus reliably stained. They are not discoloured and remain red against the blue background of the other sputum elements that are discoloured in the acid and become stained in other colours.
If bacterioscopy fails to reveal tuberculosis mycobacteria (Plate 10) in the sputum because of their scarce quantity, other techniques should be used. In luminescent microscopy a common fixed smear is coloured with a luminescing dye (rhodamine, acridine orange) and then with another stain (acid fuchsine, methylene blue), that masks the background luminescence. Luminescence of mycobacteria in the ultraviolet rays of a luminescent microscope is so bright, that mycobacteria can be seen in a dry lens (40 x). It covers a larger field of vision than the immersion lens. There exist various techniques to concentrate mycobacteria. Flotation is the most popular one. The sputum is homogenized with alkali, shaken with toluene, xylene or benzine, and mycobacteria entrapped in the droplets of the solvents float to the surface. Cream-like layer which separates on standing, is transferred by a pipette onto a warm glass, drop by drop (on one site). The preparation is allowed to dry and then fixed and stained after Ziehl-Neelsen. Another concentration method is electrophoresis. As direct current passes through the liquefied sputum, tuberculosis mycobacteria move toward the cathode. Smears are taken from the cathode and stained after Ziehl-Neelsen.
|
|