
Главная страница Случайная страница
Разделы сайта
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Chemical properties of oxides
|
|
| Basic oxides | Acid oxides |
| 1.Interaction with water | |
| Base formed: Na2O + H2O → 2NaOH CaO + H2O → Ca(OH)2 | Acid formed: SO3 + H2O → H2SO4 P2O5 + 3H2O → 2H3PO4 |
| 2. Interaction with acid or base | |
| On reactions with acid salt and water are formed tоС MgO + H2SO4 → MgSO4 + H2O tоС CuO + 2HCl → CuCl2 + H2O | On reactions with base salt and water are formed CO2 + Ba(OH)2 → BaCO3 + H2O SO2 + 2NaOH → Na2SO3 + H2O |
| Amphoteric oxides interact | |
| with acids as basic: ZnO + H2SO4 → ZnSO4 + H2O | with bases as acid: ZnO + 2NaOH → Na2ZnO2 + H2O (ZnO + 2NaOH + H2O → Na2[Zn(OH)4]) |
| 3. Interaction of basic and acid oxide with each other leads to salt formation | |
| Na2O + CO2 → Na2CO3 | |
| 4. Reduction up to simple substances: | |
| 3CuO + 2NH3 → 3Cu + N2 + 3H2O P2O5 + 5C → 2P + 5CO |
BASES. Classification… Bases - complex substances, in which atoms of metals bonded with one or several hydroxyls groups (according to electrolytic dissociation theory bases - complex substances, which under the dissociating in water solution are formed metal cations (or NH4+) and hydroxide anions OH-)!) Preparation of bases 1. Reactions of active metals (alkaline and alkaline earth metals) with water 2Na + 2H2O → 2NaOH + H2 Ca + 2H2O → Ca(OH)2 + H2 2. Interaction oxides of active metals with water BaO + H2O → Ba(OH)23. Electrolysis water solutions of salts 2NaCl + 2H2O → 2NaOH + H2 + Cl2 Chemical properties of bases
| Alkalis | Insoluble bases |
| 1. Action to indicators | |
| litmus - blue methylorange - yellow phenolphthalein – crimson | –– |
| 2. Interaction with acid oxides | |
| 2KOH + CO2 → K2CO3 + H2O KOH + CO2 → KHCO3 | –– |
| 3. Interaction with acids (reaction of neutralization) | |
| NaOH + HNO3 → NaNO3 + H2O | Cu(OH)2 + 2HCl → CuCl2 + 2H2O |
| 4. Reaction of exchange with salts | |
| Ba(OH)2 + K2SO4 → 2KOH + BaSO4↓ 3KOH + Fe(NO3)3 → Fe(OH)3↓ + 3KNO3 | –– |
| 5. Thermal decomposition | |
| –– | tоС Cu(OH)2 → CuO + H2O |
Acids. Preparation and chemical properties. Acids - complex substances, consisting from hydrogen atoms and acid radical (according to electrolytic dissociation theory: acids - electrolytes, which under the dissociating form only H+ in the capacity of cations).
ACIDS
| On composition | On hydrogen atoms number, which capable to substituted on metal |
| Oxygenless | Oxoacids (containing oxygen) | Monobasic (monoprotic) | Dibasic (diprotic) | Tribasic (triprotic) | |
| HCl, HBr, HI, H2S, HCN | HNO3, H2SO4, HClO4, H3PO4 | HCl, HNO3, HI, HClO3 (Those having one acidic proton) | H2SO4, H2SO3 H2SiO3 | H3PO4 |
Preparation of acids.1. Interaction of acid oxides with water (for oxoacids): SO3 + H2O → H2SO4 P2O5 + 3H2O → 2H3PO42. Interaction of hydrogen with non-metals and following dissolution product in water (for oxygenless acids).H2 + Cl2 → 2HCl.H2 + S → H2S3. Reactions of exchange between salt and acid Ba(NO3)2 + H2SO4 → BaSO4↓ + 2HNO3including displacement weak, flying or slightly soluble acid from its salts by means of more strong acids.Na2SiO3 + 2HCl → H2SiO3↓ + 2NaCl
| tоС | ||
| 2NaCl (hard) + H2SO4 (conc.) | → | Na2SO4 + 2HCl |
Chemical properties of acids. 1. Action to indicators. litmus – red methylorange – pink, phenolphthalein - colourless 2.Interaction with bases (reaction of neutralisation)H2SO4 + 2KOH → K2SO4+ 2H2O 2HNO3 + Ca(OH)2 → Ca(NO3)2 + 2H2O 3. Interaction with basic oxides.
| tоС | ||
| CuO + 2HNO3 | → | Cu(NO3)2 + H2O |
4. Interaction with metals. Zn + 2HCl → ZnCl2 + H2 2Al + 6HCl → 2AlCl3 + 3H2 (metals standing in the electrochemical series before hydrogen, acid-oxidizers). 5 Interaction with salts (reactions of exchange) at which stands out gas or formed residual.H2SO4 + BaCl2 → BaSO4↓ + 2HCl 2HCl + K2CO3 → 2KCl + H2O + CO2HF- Hydrofluoric acid(fluoride).HCL- Hydrochloric acid(chloride).HBr- Hydrobromic acid(bromide). HI- Hydroiodic acid(Iodide).H2S Hydrosulfuric acid(sulfide) H2SO3- Sulfurious acid(sulfite). H2SO4 Sulfuric acid(sulfate) H2CO3 Carbonic acid(carbonate) H2SiO3 Metasilicic acid (Metasilicate). HNO3 Nitric acid(Nitrate). H3PO4 Orthophosphoric acid(Orthophosphate)
Salts. Preparation and chemical properties. Salts - complex substances, which consist from atoms of metal and acid residuals. This the most multiple class of inorganic compounds.
| medium | acidic | basic | Double | mixed | Complex |
| In the time of dissociation give only metal cations (or NH4+) and anions of acid radical. Products of full substitution hydrogen atoms of acids to atoms of metals. Ex.: Na2SO4 | In the time of dissociation give only metal cations (or NH4+), hydrogen anions and anions of acid radical. Products of full substitution hydrogen atoms of multibasic acid to atoms of metal. Ex.: NaHCO3 | In the time of dissociation give only metal cations, hydroxyl anions and anions of acid radical. Products of incomplete substitution OH groups, corresponding bases to acid radicals Ex.: Mg(OH)Cl | In the time of dissociation gives two cations and one anion. Ex.: KAl(SO4)2 | Formed by means of one cation and two anions. Ex.: CaOCl2 | Contain complex cations or anions Ex.: [Ag(NH3)2]Br Na[Ag(CN)2] |
Medium salts PreparationMost of ways of getting the salts is based on the interaction of substances with opposite properties: 1) Metal with non-metal: 2Na + Cl2 → 2NaCl 2) Metal with acid: Zn + 2HCl → ZnCl2 + H2 3) Metal with solution of salt of less active metal: Fe + CuSO4 → FeSO4 + Cu 4)Basic oxide with the acid oxide: MgO + CO2 → MgCO3 6) Bases with acid oxide: Ba(OH)2 + CO2 → BaCO3↓ + H2O 7)Bases with acid: Ca(OH)2 + 2HCl → CaCl2 + 2H2O 8)Salts with the acid: MgCO3 + 2HCl → MgCl2 + H2O + CO2 BaCl2 + H2SO4 → BaSO4↓ + 2HCl 9)Bases solution with salt solution: Ba(OH)2 + Na2SO4 → 2NaOH + BaSO4↓ 10) Solutions of two salts: 3CaCl2 + 2Na3PO4 → Ca3(PO4)2↓ + 6NaCl Chemical properties 1.Thermal decomposition CaCO3 → CaO + CO2. 2Cu(NO3)2 → 2CuO + 4NO2 + O2. NH4Cl → NH3 + HCl 2. Hydrolysis Al2S3 + 6H2O → 2Al(OH)3↓ + 3H2S.FeCl3 + H2O → Fe(OH)Cl2 + HCl.Na2S + H2O → NaHS +NaOH 3.Exchange reactions with acids, bases and other salts AgNO3 + HCl → AgCl↓ + HNO3.Fe(NO3)3 + 3NaOH → Fe(OH)3↓ + 3NaNO3.CaCl2 + Na2SiO3 → CaSiO3↓ + 2NaCl 4.Oxidation-reduction reactions, stipulated by properties of cation or anion 2KMnO4 + 16HCl → 2MnCl2 + 2KCl + 5Cl2 + 8H2O Acidic salts Preparation 1.Interaction of acid with the deficit of basis.KOH + H2SO4 → KHSO4 + H2O 2.Interaction of bases with plenty acid oxides.Ca(OH)2 + 2CO2 → Ca(HCO3)2 3. Interaction of medium salts with acid.Ca3(PO4)2 + 4H3PO4 → 3Ca(H2PO4)2 Chemical properties 1.Thermal decomposition with medium salts formation.Ca(HCO3)2 → CaCO3↓ + CO2 + H2O 2. Interaction with the alkali. Reception of medium salts.Ba(HCO3)2 + Ba(OH)2 → 2BaCO3↓ + 2H2O Basic salts Preparation 1. Hydrolysis of salts, formed by weak base and strong acid.ZnCl2 + H2O → [Zn(OH)]Cl + HCl 2. Addition (by drops) a small quantities of alkalis to solutions of medium salts of metals.AlCl3 + 2NaOH → [Al(OH)2]Cl + 2NaCl 3. Interaction of weak acids salts with medium salts.2MgCl2 + 2Na2CO3 + H2O → [Mg(OH)]2CO3↓ + CO2 + 4NaCl Chemical properties 1. Interaction with the acid: formation of medium salts. Sn(OH)Cl + HCl → SnCl2 + H2O.
Laboratory equipment. laboratory apparatus (laboratory equipment) лабораторное оборудование; Bunsen burner горелка Бунзена; Desiccator эксикатор (сушилка) Used for store material and protect it from air contamination or humidity; air regulator регулятор поступления воздуха; bench torch настольная горелка; oxygen inlet подвод кислорода; funnel воронка; beaker стакан-Used for measuring liquid roughly volume with low accuracy; burette(Used for titration where titrant pored inside) (for delivering measured quantities of liquid) бюретка (для выпуска измеренных объемов жидкости); burette stand штатив для бюреток; burette clamp зажим для бюреток; graduated pipette градуированная пипетка; pipette пипетка- Used for transfer exact amount of liquid; measuring cylinder (measuring glass) мерный цилиндр (измерительный стакан) Used for measuring liquid with better accuracy than beaker; measuring flask мерная колба Used for measuring liquid with high accuracy; test tube пробирка; test tube rack штатив для пробирок; flat-bottomed flask плоскодонная колба; ground glass neck горлышко с притертой стеклянной пробкой; long-necked round-bottomed flask длинногорлая круглодонная колба; cylinder цилиндр; Mortar and Pestle- Used for graining materials which have large particle size to small.; Stand (штатив)Used commonly as base for holding distillation system and burette along with clamp and boss head; Watch glass Used for air drying or oven drying of liquid; Erlenmeyer flask (conical flask)Used for titration or filtration of liquids and to prevent air contamination to sample during work
LABORATORY SAFETY RULESA chemical laboratory can be a hazardous place to work if common safety rules are not enforced. If basic rules are strictly enforced, the chance of one being injured becomes very small. When working you will still need to use electric equipment, hot water, and concentrated solutions. These are only safe to work with if you follow the correct procedures. With proper understanding of what you are doing, careful attention to safety precautions, and adequate supervision, you will fi nd the chemical laboratory to be a safe place in which you can learn much about chemistry. Some general precautions and procedures applicable to any chemical laboratory are summarized below.1.Be prepared to work when you arrive at the laboratory. Read the experiment BEFORE you come to lab. Make sure you fully understand the experiment before starting the actual work. If you have a question, ask your professor/teacher/laborant for clarification BEFORE starting the procedure.2.Carefully follow directions, both written and oral. Do only the steps described in the procedure of the experiment or that are described and/or approved by the teacher. If you are in doubt about any procedure, ask your teacher for help.3.Everyone should be alert and proceed with caution at all times in the laboratory. Take care not to bump another student and to remain at your lab station while performing an experiment. An unattended experiment can result in an accident.4.Wear safety glasses/goggles whenever you are in the lab. Aprons are required for some experiments. You may wear aprons at your discretion for any experiment to avoid staining or ruining your clothing. 5.A lab coat must be wear. Protective gloves should be worn whenever the potential exists for contact with toxic chemicals. Shorts and sandals are not safe lab attire, since they provide no protection from splashed or spilled materials. Bare feet are absolutely forbidden in the laboratory. To avoid entanglement with laboratory equipment, necklaces and bracelets should not be worn.6.Locate the emergency evacuation plan posted by the door. Know your exit routes! 7.Locate emergency eyewash station, fire extinguisher, fire alarm, and fire blanket. 8.Dispose of all broken glassware in the proper receptacle. Never put broken glass in the trash can.9.Do only the experiments that have been assigned by your teacher. No unauthorized experiments will be allowed.10.Notify your instructor immediately if you are injured in the laboratory; no matter how slight. 11.NO FOOD OR DRINK IS ALLOWED IN THE LABORATORY. Never put anything into your mouth while you are in the laboratory. Eating, drinking, and smoking are strictly forbidden in the laboratory!!! Never pipette fluids by mouth. Check odors cautiously. Never taste a chemical. 12.Shoes must be worn in the laboratory. These shoes must fully enclose your foot.13DO NOT WEAR CONTACT LENSES IN THE LABORATORY!!! Approved eye protection (safety glasses with side shields or goggles) must be worn at all times in the laboratory. Contact lens must never be worn because they can trap corrosive, volatile materials which may damage the eye.14.Long hair must be tied up in a bun during lab work. Loose long sleeves should be avoided in the lab. It is a fire hazard!!!
Coordination compounds. Classification, structure, nomenclature, properties.Transition metal ions characteristically form coordination compounds, which are usually colored and often paramagnetic. A coordination compound typically consists of a complex ion, a transition metal ion with its attached ligands, and counter ions, anions or cations as needed to produce a compound with no net charge.Structure of coordination compounds.
| K4[Fe(CN)6] – potassium hexacyanoferrate (II) | |
| K4[Fe(CN)6] | – External sphere |
| K4[Fe(CN)6] | – Internal sphere |
| K4[Fe(CN)6] | – Central atom |
| K4[Fe(CN)6] | – Coordinate relation |
| K4[Fe(CN)6] | – Ligand |
Central, complex forming, atoms is usually serves ions of metals of greater periods (Co, Ni, Pt, Hg, Ag, Cu). Typical ligands are OH-, CN-, NH3, CO, H2O; they connected with the central atom by donor-acceptor bound. Chemical properties 1.Destruction of complexes at the expense of forming the slightly-soluble compounds2[Cu(NH3)2]Cl + K2S → CuS↓ + 2KCl + 4NH3 2. The exchange of ligands between external and internal spheresK2[CoCl4] + 6H2O → [Co(H2O)6]Cl2 + 2KCl. Naming Coordination Compounds A complex is a substance in which a metal atom or ion is associated with a group of neutral molecules or anions called ligands. Coordination compounds are neutral substances (i.e. uncharged) in which at least one ion is present as a complex. The coordination compounds are named in the following way. А. To name a coordination compound, no matter whether the complex ion is the cation or the anion, always name the cation before the anion. (This is just like naming an ionic compound.) B. In naming the complex ion: 1. Name the ligands first, in alphabetical order, then the metal atom or ion. Note: The metal atom or ion is written before the ligands in the chemical formula. 2. The names of some common ligands are listed in Table 1. For anionic ligands end in " -o"; for anions that end in " -ide" (e.g. chloride), " -ate" (e.g. sulfate, nitrate), and " -ite" (e.g. nirite), change the endings as follows: -ide  -o; -ate
-o; -ate  -ato; -ite -ito For neutral ligands, the common name of the molecule is used e.g. H2NCH2CH2NH2 (ethylenediamine). Important exceptions: water is called ‘aqua’, ammonia is called ‘ammine’, carbon monoxide is called ‘carbonyl’, and the N2 and O2 are called ‘dinitrogen’ and ‘dioxygen’ПРИМЕР:. K4[Fe(CN)6] Answer: potassium hexacyanoferrate (II)
-ato; -ite -ito For neutral ligands, the common name of the molecule is used e.g. H2NCH2CH2NH2 (ethylenediamine). Important exceptions: water is called ‘aqua’, ammonia is called ‘ammine’, carbon monoxide is called ‘carbonyl’, and the N2 and O2 are called ‘dinitrogen’ and ‘dioxygen’ПРИМЕР:. K4[Fe(CN)6] Answer: potassium hexacyanoferrate (II)
Basic (fundamental) chemical laws - Law of conservation of mass. We use this law everytime when balancing the chemical equations to show that weight of the reacting substances is equal to the mass of substances formed by the reaction. For example: 2 H2 + O2 = 2 H2O Law of conservation of energy. Physical principle that the total amount of energy in a system remains constant, although energy can be changed from one form to another or transferred from one object to another. (Other formulation: energy can be converted from one form to another, but can be neither created nor destroyed). We will defi ne energy as the capacity to do work or to produce heat. Laws of defi nite and multiple proportions. Joseph Proust (1754–1826), showed that a given compound always contains exactly the same proportion of elements by mass.When two elements form a series of compounds, the ratios of the masses of the second element that combine with 1 gram of the fi rst element can always be reduced to small whole numbers. Avogadro’s law Avogadro’s law, a statement that under the same conditions of temperature and pressure, equal volumes of different gases contain an equal number of molecules. This empirical relation can be derived from the kinetic theory of gases under the assumption of a perfect (ideal) gas. The law is approximately valid for real gases at sufficiently low pressures and high temperaturesThe specific number of molecules in one gram-mole of a substance, defined as the molecular weight in grams, is 6.02214129 × 1023, a quantity called Avogadro’s number
Basic gas laws.Boyle's law (sometimes referred to as the Boyle–Mariotte law): at constant temperature the volume of a gas is inversely proportional to its pressure.  T = const Inverse proportionality occurs when one variable gets larger by the same factor as another gets smaller. Charles’s Law: at constant volume the pressure of a gas is directly proportional to its temperature.
T = const Inverse proportionality occurs when one variable gets larger by the same factor as another gets smaller. Charles’s Law: at constant volume the pressure of a gas is directly proportional to its temperature. 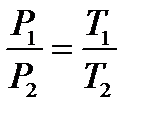 V = const.Charles defi ned a new temperature scale in which the lowest possible temperature is 0оС absolute, corresponding to -273.15 оС This temperature is called absolute zero. The symbol T is used for absolute temperature, and t is used for Celsius temperature: T = t + 273.15 Temperatures of approximately 0.000001 K have been produced in laboratories, but 0 K has never been reached. Gay-Lussac's Law: at constant pressure the volume of a gas is directly proportional to its temperature.
V = const.Charles defi ned a new temperature scale in which the lowest possible temperature is 0оС absolute, corresponding to -273.15 оС This temperature is called absolute zero. The symbol T is used for absolute temperature, and t is used for Celsius temperature: T = t + 273.15 Temperatures of approximately 0.000001 K have been produced in laboratories, but 0 K has never been reached. Gay-Lussac's Law: at constant pressure the volume of a gas is directly proportional to its temperature. 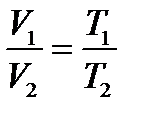 P = const A temperature of 0оC and a pressure of 1.00 atm (760 mm Hg, 101.3 kPa) constitute a set of conditions for a gas often called standard temperature and pressure (abbreviated STP). Combined gas law. The combined gas law is a gas law that combines Charles's law, Boyle's law, and Gay-Lussac's law. There is no official founder for this law; it is merely an amalgamation of the three previously discovered laws.The combined gas law clearly states that: the ratio between the pressure-volume product and the temperature of a system remains constant. This can be stated mathematically as
P = const A temperature of 0оC and a pressure of 1.00 atm (760 mm Hg, 101.3 kPa) constitute a set of conditions for a gas often called standard temperature and pressure (abbreviated STP). Combined gas law. The combined gas law is a gas law that combines Charles's law, Boyle's law, and Gay-Lussac's law. There is no official founder for this law; it is merely an amalgamation of the three previously discovered laws.The combined gas law clearly states that: the ratio between the pressure-volume product and the temperature of a system remains constant. This can be stated mathematically as 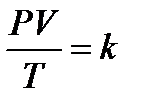 where: P is the pressure; V is the volume; T is the temperature measured in Kelvin; k is a constant (with units of energy divided by temperature).For comparing the same substance under two different sets of conditions, the law can be written as:
where: P is the pressure; V is the volume; T is the temperature measured in Kelvin; k is a constant (with units of energy divided by temperature).For comparing the same substance under two different sets of conditions, the law can be written as: 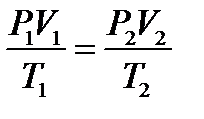
MOLE. MOLAR MASS. In chemical processes involve the smallest particles - molecules, atoms, ions, electrons. The number of these particles even in small quantity of substances is very large. Therefore, to avoid mathematical operations with large numbers, to characterize the quantity of a substance involved in a chemical reaction, a special unit is used - the mole. Mole - is the amount of the substance, which contains a number of particles (molecules, atoms, ions), equal to the Avogadro's constant: NA = 6.0221367·1023 mol-1 This number is about the number of grains of sand that would fi t into a sphere the size of the Earth! Remember the value of this number to at least three signifi cant digits. Avogadro’s constant (or Avogadro’s number) NA is defined as the number of atoms contained in 12 g of carbon isotope 12C: 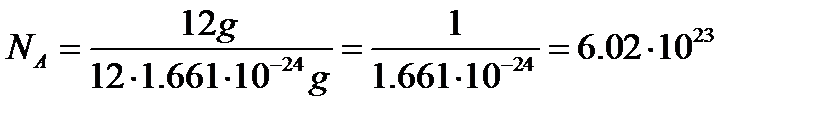 Thus, one mole of substance contains 6.02 • 1023 particles of this substance. Any amount of a substance can be expressed as a certain number of moles of ν (nu):
Thus, one mole of substance contains 6.02 • 1023 particles of this substance. Any amount of a substance can be expressed as a certain number of moles of ν (nu): 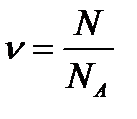 where N – number of particles of the substance; NA – Avogadro’s constant. The molar mass of the substance (M) – is the mass of 1 mole of the substance. The molar mass of any substance can be calculated, when mass (m) and amount of substance (ν) are known:
where N – number of particles of the substance; NA – Avogadro’s constant. The molar mass of the substance (M) – is the mass of 1 mole of the substance. The molar mass of any substance can be calculated, when mass (m) and amount of substance (ν) are known: 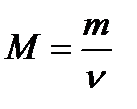 Accordingly, knowing the mass and molar mass of the substance, it is possible to calculate the number of moles:
Accordingly, knowing the mass and molar mass of the substance, it is possible to calculate the number of moles: 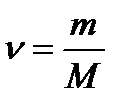 or find the weight of substance from the number of moles and molar mass: m = ν • MThus, mole is a quantity of a substance containing the same number of particles, but having a different weight for the different substances, because substance particles (atoms and molecules) has different weight. For example: calculation the molar mass of nitric acid HNO3.Mr (HNO3) = Ar (H) + Ar (N) + 3·Ar (O) = 1 + 14 + 3·16 = 63 g/mol.
or find the weight of substance from the number of moles and molar mass: m = ν • MThus, mole is a quantity of a substance containing the same number of particles, but having a different weight for the different substances, because substance particles (atoms and molecules) has different weight. For example: calculation the molar mass of nitric acid HNO3.Mr (HNO3) = Ar (H) + Ar (N) + 3·Ar (O) = 1 + 14 + 3·16 = 63 g/mol.
Atomic structure. Dalton’s atomic theory. Atomos, the Greek root of the word atom, means “indivisible.” It was originally believed that the atom was the ultimate indivisible particle of which all matter was composed. Lord Rutherford showed in 1911 that the atom is not homogeneous, but rather has a dense, positively charged center surrounded by electrons. Subsequently, scientists have learned that the nucleus of the atom can be subdivided into particles called neutrons and protons. In fact, in the past two decades it has become apparent that even the protons and neutrons are composed of smaller particles called quarks. For most purposes, the nucleus can be regarded as a collection of nucleons (neutrons and protons), and the internal structures of these particles can be ignored. The number of protons in a particular nucleus is called the atomic number (Z), and the sum of the neutrons and protons is the mass number (A). Atoms that have identical atomic numbers but different mass number values are called isotopes. In 1803, John Dalton (1766–1844) proposed his atomic theory, including the following postulates. 1. Matter is made up of very tiny, indivisible particles called atoms.2. The atoms of each element has mass, but the mass of the atoms of one element is different from the mass of the atoms of every other element. 3. Atoms combine to form molecules. When they do so, they combine in small, whole-number ratios 4. Atoms of some pairs of elements can combine with each other in different small, whole-number ratios to form different compounds.5. If atoms of two elements can combine to form more than one compound, the most stable compound has the atoms in a 1: 1 ratio. (This postulate was quickly shown to be incorrect.) The postulates of Dalton’s atomic theory explained the laws of chemical combination very readily. 1. The law of conservation of mass is explained as follows: Because atoms only exchange “partners” during a chemical reaction and are not created or destroyed, their mass is also neither created nor destroyed. This, mass is conserved during a chemical reaction. 2. The law of defi nite proportions is explained as follows: Because atoms react in definite integral ratios (postulate 3), and atoms of each element have a definite mass (postulate 2), the mass ratio of one element to the other(s) must also be definite. 3. The law of multiple proportions is explained as follows: Because atoms combine in different ratios of small whole numbers (postulate 4), for a given number of atoms of one element, the number of atoms of the other element is in a small, whole-number ratio. A given number of atoms of the fi rst element implies a given mass of that element, and a small, whole-number ratio for the atoms of the second element (each of the same mass) implies a small, whole-number ratio of masses of the second element.
The Periodic system and Periodic law. The classic Periodic Law formulation of D.I.Mendeleev is as states: the properties of elements and therefore the properties of the simple and complex substances they form are in the periodic dependence on their atomic weight. Nowadays the formulation is: the properties of elements and therefore the properties of the simple and complex substances they form are in the periodic dependence on the charge of the atomic nuclei. letters in the boxes are the symbols for the elements; these abbreviations are based on the current element names or the original names. The number shown above each symbol is the atomic number (number of protons) for that element. For example, carbon (C) has atomic number 6, and lead (Pb) has atomic number 82. Most of the elements are metals. Metals have characteristic physical properties such as effi cient conduction of heat and electricity, malleability (they can be hammered into thin sheets), ductility (they can be pulled into wires), and (often) a lustrous appearance. Chemically, metals tend to lose electrons to form positive ions. For example, copper is a typical metal. An element block is a set of elements located in adjacent element groups. Element blocks are named for their characteristic orbital, which is determined by the highest energy electrons: s-block First two groups of the periodic table -- alkali metals and alkaline earths p-block Last six element groups of the periodic table, excluding helium. The p-block elements include all of the nonmetals except for hydrogen and helium, the semimetals, and the post-transition metals. d-block Transition metals of element groups 3-12. f-block Inner transition elements, usually the lanthanide and actinide series, including lanthanum and actinium. g-block (proposed) Would be expected to include element with atomic numbers higher than 118.
Periodic Trends in Atomic Properties. Periodic trends are specific patterns that are present in the periodic table, which illustrate different aspects of a certain element, including its size and its properties with electrons. The main periodic trends include: electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. The periodic trends that arise from the arrangement of the periodic table provide chemists with an invaluable tool to quickly predict an element's properties. These trends exist because of the similar atomic structure of the elements within their respective group families or period and the periodic nature of the elements. Electronegativity Trends Electronegativity measures an atom's strength to attract and form bonds with electrons. This property exists due to the electronic configuration of atoms. 1. As you move to the right across a period of elements, electronegativity increases.; 2.As you move down a group, electronegativity decreases.; 3.Important exceptions of the above rules include the noble gases, lanthanides, and actinides.; 4.As for the transition metals, while they have values, there is little variance among them as you move across the period and up and down a group. Ionization Energy Trends Ionization Energy is the amount of energy required to remove an electron from a neutral atom in its gaseous phase. Thus, ionization energy increases from left to right on the periodic table. 1. The ionization energy of the elements within a period generally increases from left to right. This is due to valence shell stability. 2. The ionization energy of the elements within a group generally decreases from top to bottom. This is due to electron shielding. 3. The noble gases possess very high ionization energies because of their full valence shell. Note that Helium has the highest ionization energy of all the elements. Electron Affinity Trends Like the name suggests, electron affinity describes the ability of an atom to accept an electron. This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to right across a period. 1. Electron affinity increases from left to right within a period. This is caused by the decrease in atomic radius. 2. Electron affinity decreases from top to bottom within a group. This is caused by the increase in atomic radius.
Chemical bond. Mechanisms of formation. Basic characteristics of the chemical bond. A chemical bond is an attraction between atoms that allows the formation of chemical substances that contain two or more atoms. Basic characteristics of the chemical bond are: bond energy, bond length and bond angle. The bond energy is a measure of the amount of energy needed to break apart one mole of covalently bonded gases. The SI units used to describe bond energy is kilojoules per mole of bonds (kJ/mol). Bond length is the distance between the centers of two covalently bonded atoms. The length of the bond is determined by the number of bonded electrons (the bond order). Generally, the length of the bond between two atoms is approximately the sum of the covalent radii of the two atoms, X + Y.Bond length is given in picometers. Therefore, the bond length of a triple bond < double bond < single bond. Bond angle is the angle that is formed between two adjacent bonds on the same atom. Covalent Bonding. Metal atoms can donate electrons to nonmetal atoms, but nonmetal atoms do not form monatomic positive ions because they would have to donate too many valence electrons to form octets. (Single nonmetal atoms do not donate electrons at all, but some groups of nonmetal atoms can. This will be discussed later in this section. Nonmetal atoms can accept electrons from metal atoms if such atoms are present; otherwise, they can attain an octet by electron sharing. A covalent bond consists of shared electrons. One pair of electrons shared between two atoms constitutes a single covalent bond, generally referred to as a single bond. An unshared pair of valence electrons is called a lone pair. Elements or compounds bonded only by covalent bonds form molecules. Donor-Acceptor Bond (DAB) – special type of covalent bond DAB (also coordination bond), a term denoting one of the ways in which a chemical covalent bond is formed. The ordinary covalent bond between two atoms is due to the interaction of two electrons, one from each atom. The donor-acceptor bond is formed by a pair of electrons from one atom (the donor) and a free (unfilled) orbital from another (the acceptor). Hydrogen bonding is the intermolecular force of attraction between a hydrogen atom in one molecule and a small, highly electronegative atom with an unshared pair of electrons in another molecule. Metallic bonding Metallic bonding is the electromagnetic interaction between delocalized electrons, called conduction electrons, gathered in an " electron sea", and the metallic nuclei within metals. Understood as the sharing of " free" electrons among a lattice of positively charged ions (cations), metallic bonding is sometimes compared with that of molten salts; however, this simplistic view holds true for very few metals.
Oxydation-reduction reactions (redox-reactions). Reactions, in which one or more electrons are transferred, are called oxidation–reduction reactions or redox reactions.We defi ne the oxidation states (or oxidation numbers) of the atoms in a covalent compound as the imaginary charges the atoms would have if the shared electrons were divided equally between identical atoms bonded to each other or, for different atoms, were all assigned to the atom in each bond that has the greater attraction for electrons.Oxidation–reduction reactions are characterized by a transfer of electrons. In some cases, the transfer occurs in a literal sense to form ions.
| OXIDIZED | REDUCED |
| Loses electrons | Gains electrons |
| Oxidation state increases | Oxidation state decreases |
| Redusing agent | Oxidising agent |
Types of chemical reactions. 1. Decomposition Reactions – реакции разложения A compound breaks into parts. compound → element + element 2H2O → 2H2 + O2
2. Synthesis Reactions – реакции присоединения (синтеза) Elements are joined together.
element + element → compound 2H2 + O2 → 2H2O Compounds are joined together compound + compound → compound 6CO2 + 6H2O → C6H12O6 + 6O2 3. Single Displacement Reactions – реакции единичного замещения A single element replaces an element in a compound. element + compound → element + compound Zn + 2HCl → H2 + ZnCl2 4. Double Displacement Reactions – реакции двойного замещения An element from each of two compounds switch places. compound + compound → compound + compound H2SO4 + 2NaOH → Na2SO4 + 2H2O 5. Combustion Reactions – реакции горения A hydrocarbon (a compound containing only carbon and hydrogen) combines with oxygen. The products of combustion are always carbon dioxide and water. hydrocarbon + oxygen → carbon dioxide + water CH4 + 2O2 → CO2 + 2H2O When metallic substances combine with oxygen, the result is an oxidation-reduction reaction. The rusting of iron - 4Fe + 3O2 → 2Fe2O3Chemical reactions can be classified in other ways as well: Neutralization Reactions – реакция нейтрализации - Special types of double displacement reactions that involve the reaction between an acid and base to form a salt and water.acid + base → salt + water A suspension of solid magnesium hydroxide in water is widely used as an antacid to neutralize excess stomach acid: Mg(OH)2 (s) + 2HCl (aq) → MgCl2 (aq) + 2H2O Oxidation-Reduction Reactions – окислительно-восстановительные реакции Any reaction in which elements experience a change in oxidation number. one atom gains e-minus and another atom looses e-minus S + O2 → SO2 In the reaction above, sulfur and oxygen both have an oxidation number of zero before the reaction. After the reaction, sulfur is +4 and oxygen is − 2. Precipitation Reactions – реакции осаждения. Aqueous reactions that involve the formation of a precipitate (solid). soluble compound + soluble compound → insoluble compound 2KI (aq) + Pb(NO3)2 (aq) → 2KNO3 (aq) + PbI2 (s) ↓ The physical state symbol (aq) says the reaction is taking place in a water solution. The physical state symbol (s) says the lead (II) iodide is a solid - therefore insoluble in the solution. Hydrolysis (гидролиз) is a reaction involving the breaking of a bond in a molecule using water.
Solutions. Concentration units. Solution - a mixture consisting of a solute and a solvent. Solute - component of a solution present in the lesser amount. Solvent - component of a solution present in the greater amount. Concentration - amount of a solute present in a solution per standard amount of solvent There are numerous ways of expressing concentrations. It will be important to know the units used to express each concentration, as these units essentially define the concentration. Percent by Mass (Weight percent, ω %) The percent by mass of a solute is simply the number of grams of solute in exactly 100 g of solution. It can be calculated as exactly 100% times the mass of the solute divided by the mass of the entire solution. Molality (m) Molality is often used as the concentration unit involved in calculations dealing with colligative properties, such as freezing point depression, boiling point elevation and osmotic pressure. Molarity (M) Chemical reactions often take place when two solutions are mixed. To perform stoichiometric calculations in such cases, we must know two things: (1) the nature of the reaction, which depends on the exact forms the chemicals take when dissolved, and (2) the amounts of chemicals present in the solutions, usually expressed as concentrations. The concentration of a solution can be described in many different ways. At this point we will consider only the most commonly used expression of concentration, molarity (M), which is defi ned as moles of solute per volume of solution in liters: 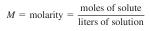 Normality (molar concentration of equivalent, N, Ceq) Another concentration measure sometimes encountered is normality (symbolized by N). Normality is defi ned as the number of equivalents per liter of solution, where the defi nition of an equivalent depends on the reaction taking place in the solution
Normality (molar concentration of equivalent, N, Ceq) Another concentration measure sometimes encountered is normality (symbolized by N). Normality is defi ned as the number of equivalents per liter of solution, where the defi nition of an equivalent depends on the reaction taking place in the solution
Fundamentals of organic chemistry Organic chemistry is the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. In chemistry, hybridisation (or hybridization) is the concept of mixing atomic orbital’s into new hybrid orbitals (with different energies, shapes, etc., than the actual orbital’s hybridizing) suitable for the pairing of electrons to form chemical bonds in valence bond theory.Types of hybridization sp3 hybridsHybridisation describes the bonding atoms from an atom's point of view. That is, for a tetrahedrally coordinated carbon (e.g., methane CH4), the carbon should have 4 orbitals with the correct symmetry to bond to the 4 hydrogen atoms.sp2 hybridsOther carbon based compounds and other molecules may be explained in a similar way as methane. For example, ethene(C2H4) has a double bond between the carbons.sp hybridsThe chemical bonding in compounds such as alkynes with triple bonds is explained by sp hybridization
Classification of organic substances. The number of different design possibilities for organic molecules is endless. In order to enable classification of such a large number of molecules, organic chemists have employed the principle of classifying all organic compounds into families according to their functional groups. This greatly simplifies the study of organic compounds as molecules with the same functional groups behave the same in most chemical reactions. Alkanes -This compounds consists of substances that contain only carbon and hydrogen joined by single bonds. Alkenes- Hydrocarbons that contain a carbon-carbon double bond. Alkynes-Hydrocarbons that contain a carbon-carbon triple bond. Alkadienes -Hydrocarbons that contain a two carbon-carbon double bonds Alkanoles (alcohols). Compounds which contains the functional group -OH. Alkanoic acids (carboxylic acids)- These compounds contain a functional group known as the carboxyl group –COOH Amides-Compounds which contains the functional group –CONH2 Amines-Amines are organic compounds that contain nitrogen that is bonded to only a carbon or a hydrogen atom. Aromatic These are compounds that contain the benzene ring as part of their structure.
|
|