
Главная страница Случайная страница
Разделы сайта
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Tadalafil (any dose: fixed or flexible) versus placebo.
|
|
Harms. The occurrence of total and serious adverse events across the 23 placebo-controlled trials was reported poorly.215–227, 229, 230, 233–240
In the majority of these trials, the frequency of any adverse events (i.e., the proportion of patients with at least one adverse event) was greater either numerically or with statistical significance in the tadalafil arms than in the placebo arms.215, 220, 222, 223, 225–227, 229, 230, 236–240
For example, in one trial, the proportion of patients who experienced at least one adverse event in the tadalafil and placebo arms were 51.7 versus 26.5 percent, respectively (p < 0.001).222 In another trial, 225 the corresponding numbers were 40 (22.5 percent) versus two (1.3 percent) (p value was not reported). Even though the proportion of patients in one trial226 was numerically greater in the tadalafil arms (39.7–44.4 percent) than in the placebo arm (31.0 percent), this difference was not statistically significant (p = 0.247). Most common adverse events reported across all trials were headache, back pain, dyspepsia, dizziness, nasal congestion, flushing, and myalgia. In general, the occurrence of these events tended to be numerically more frequent in tadalafil arms than in placebo arms. Moreover, a statistically significant higher incidence of these events was reported across several trials in tadalafil versus placebo arms.215, 220, 222, 223, 225, 226, 239 The majority of the trials reported that tadalafil was well tolerated and that patients had had adverse events mostly of mild or moderate severity.
Eleven of the 23 trials did not report whether there had been any occurrence of serious adverse events.216, 218, 219, 221, 225–227, 229, 230, 237, 239
In the remaining 12 trials, 215, 217, 220, 222–224, 233–236, 238, 240 the incidence of serious adverse events (i.e., the proportion of patients with at least one serious adverse event) was reported to have been about 5.0 percent222 or less, or 0, 238, and to have been similar in tadalafil and placebo arms.215, 217, 220, 222–224, 233, 235, 240 In one trial, 217, 243 three patients who received tadalafil developed carotid artery bruit, esophageal spasm, and brain neoplasm (one case of each event).217 Other specific serious adverse events that were reported were single cases of pulmonary embolism and subarachnoid hemorrhage, 223 two cases of chest pain requiring hospital admission, 224 and one case of worsening CAD.236 In one trial, 235 five patients experienced at least one of the following serious adverse events in the tadalafil arm: acute myocardial infarction (AMI), benign lung neoplasm, back pain, road traffic accident, and pancreatitis. Two trials215, 234 reported one death each which had occurred due to AMI215 and cardiac arrest.234 Of the 12 trials that reported any occurrence of serious adverse events, three trials215, 220, 222 did not specify what these events were.
The proportion of patients who withdrew due to adverse events across trials was five–six percent217, 222, 224 or less and similar across the tadalafil and placebo arms.215–220, 222–227, 229, 230, 233–240
Efficacy. In general, the results of the 23 placebo-controlled trials showed that patients who received tadalafil (10 or 20 mg) experienced greater improvement in erectile functioning (e.g. outcomes based on responses to IIEF, SEP, and GAQ) compared with those who received placebo. The observed between- or within-arm differences in the mean endpoint scores/mean score changes for the IIEF “EF” domain216, 217, 219, 220, 222–224, 226, 227, 229, 234, 236–240 and for the per-patient proportion of “yes” answers to the SEP questions 2–3, were statistically significant (p value < 0.05).215–217, 219, 220, 222–227, 229, 230, 234, 236–240Similarly, the proportion of patients who answered “yes” to the GAQ-Q1/2 was statistically significantly greater in tadalafil than in placebo arms.216, 217, 220, 222–224, 226, 227, 229, 234, 236–240
For example, the mean within-arm IIEF “EF” domain absolute score change observed in tadalafil arms (10 or 20 mg dose) across trials216, 217, 219, 220, 222–224, 226, 227, 229, 234, 236–240 ranged from 5.2222 to 12.0, 219 whereas the corresponding treatment response observed in placebo arms ranged from -1.6216, 242 to 2.9.219 The mean change in the proportion who responded “yes” to SEP Q3 (i.e., per-patient percentage of successful intercourse attempts) in tadalafil arms (10 or 20 mg dose) ranged from 23.0 percent222 to 56.5 percent.220, 237 The corresponding mean treatment response change in placebo arms ranged from 0.9 percent216 to 18.3 percent.237 The proportion of patients who responded “yes” to GAQ-Q1 in tadalafil arms (10 or 20 mg) across trials216, 217, 220, 222–224, 226, 227, 229, 234, 236–240 ranged from 62.0 percent222 to 92.3 percent.229 For placebo arms this proportion across the same trials ranged from 12.8 percent216 to 54.5 percent.229
In a parallel-arm trial of patients with LUTS (65 percent ED patients), 233 138 patients (n = 99 ED patients) received the dose-escalated tadalafil (5 mg for 6 weeks and the 5–20 mg dose-escalation for another 6 weeks) and, compared with those receiving placebo, had a statistically significant greater mean change in the IIEF “EF” domain score at 6 and 12 weeks of treatment (12 weeks: 7.7 versus 1.4, p < 0.001). Furthermore, results of two trials235, 238 indicated that patients receiving even lower doses of tadalafil (2.5 mg and 5 mg) compared with those in the placebo group had greater statistically significant improvements in erectile functioning with respect to the mean score changes in the IIEF “EF” domain (19.1–20.8 versus 14.6, p < 0.001) and the per-patient proportion of “yes” answer to SEP Q2–3 (for Q2: 65.3–70.7 versus 51.1, p < 0.001 and for Q3: 50.0–57.0 versus 31.3, p < 0.001).235 Compared with the placebo group, in both tadalafil dose-groups there was a significantly greater proportion of patients who answered “yes” to GAQ1–2 (5 mg: 70.7–72.8 percent, versus 2.5 mg: 58.5–62.8 percent, versus placebo: 23.9–26.1 percent).235
Furthermore, patients who received tadalafil compared with those who received placebo had statistically significant greater improvements in erectile function as measured by the mean IIEF score change from baseline to endpoint for the IIEF “Intercourse Satisfaction” and “Overall Satisfaction” domains.216, 217, 223, 224, 226, 227, 229, 234–240, 243
In several trials, there was a statistically significant greater mean per-patient percentage of successful intercourse attempts measured at different intervals after dosing in tadalafil arms compared with placebo arms.217, 219, 220, 224, 225, 230
The mean overall EDITS score in patients who received tadalafil showed a statistically significant improvement compared with that in patients who received placebo.217, 222, 223 The mean overall EDITS score values in tadalafil arms across the trials were 66.8, 217, 243 58.0, 222 and 77.223 The corresponding values for placebo arms in these trials were 35.6, 34.0, and 46.0, respectively. In one of these trials, 223 patients treated with tadalafil had a median EDITS score of 84, (95 percent CI: 80–86) as opposed to those treated with placebo, who had a median EDITS score of 41 (95 percent CI: 32–59). The difference between the two median scores was statistically significant (p < 0.001).
The mean change measured on individual IIEF Q3–4 scores (Q3, penetration ability; Q4, maintenance ability) was reported in 8 trials (out of the 23 placebo-controlled trials), 217, 220, 226, 234–237, 240 all of which showed highly statistically significant improvements for patients treated with tadalafil patients compared with those treated with placebo (p < 0.001).
The authors of one trial218 showed that after a 4-week therapy, patients treated with tadalafil experienced greater improvements in endothelial function as measured by brachial artery flow-mediated dilation (FMD) than patients treated with placebo. For example, in the tadalafil arm, the mean change in FMD from baseline to the end point was statistically significant (9.3 versus 4.2 percent, p < 0.01), whereas in the placebo arm the mean FMD did not change (4.1 versus 4.3, p > 0.05).
Four studies examined the efficacy according to severity of ED.216, 235, 237, 238 The number of patients achieving normal scores in IIEF-EF were higher in mild ED compared with moderate or severe ED.216, 235, 237, 238 Similarly higher end scores of IIEF-EF were achieved in patients with mild compared with moderate or severe ED.216, 235, 237, 238 (Table 11)
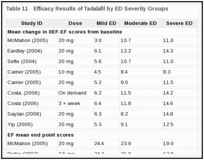
|
|