
Главная страница Случайная страница
Разделы сайта
АвтомобилиАстрономияБиологияГеографияДом и садДругие языкиДругоеИнформатикаИсторияКультураЛитератураЛогикаМатематикаМедицинаМеталлургияМеханикаОбразованиеОхрана трудаПедагогикаПолитикаПравоПсихологияРелигияРиторикаСоциологияСпортСтроительствоТехнологияТуризмФизикаФилософияФинансыХимияЧерчениеЭкологияЭкономикаЭлектроника
Organic compounds containing oxygen Monocarboxylic acids.
|
|
C1-HCOOH-Methanoic acid-Formic acid (Common name) -(formiate); C2-CH3COOH-Ethanoic acid-Acetic acid-(acetate); C3-CH3CH2COOH-Propanoic acid-Propionic acid-(propionate; C4-CH3(CH2)2COOH-Butanoic acid-Butyric acid- (butyrate) C5-CH3(CH2)3COO-Pentanoic acid-Valeric acid-(valerate); C6-CH3(CH2)4COOH-Hexanoic acid-Caproic acid-(caproate) ДИКАРБОНОВЫЕ C2-HOOC-COOH-Ethanedioic acid-Oxalic acid-Oxalate C3-HOOC-CH2-COOH-Propanedioic acid-Malonic acid-Malonate; C4-HOOC-(CH2)2-COO-Butanedioic acid-Succinic acid-Succinate; C5-HOOC-(CH2)3-COOH-Pentanedioic acid-Glutaric acid-Glutarate. ESTERS This name is given to those generally smelling substances that contain the esters functional group R-COO-R1. The sweet and pleasant odours and tastes of many foods are due to complex mixtures of organic compounds, of which esters are generally the most prevalent component. Table lists some of the esters that are used as flavouring agents.Пример methyl butanoate, ethyl butanoate, butyl butanoate. ETHERS These are substances that contain two organic groups attached to the same OXYGEN atom. i.e. They contain the functional group - R – O – R’ The R groups may be aromatic or aliphatic or they may be part of a ring structure.
Types of organic reactions- A chemical change involves a reorganization of the atoms in one or more substances. This process is represented by a chemical equation with the reactants on the left side of an arrow and the products on the right side 1. Addition. The double bond dissolves back to single bond and new bonds reach out to A and B whose bond is also dissolving. AE – electrophylic addition – электрофильное присоединение AN – nucleophylic addition – нуклеофильное присоединение. 2. Elimination (E) This is the opposite of addition i.e. a double bond is created when two groups on adjacent carbons are rejected by the molecule. The groups are just those groups which were added in the first place. The only one we cannot do is the elimination of H-H bond. 3. Substitution (S) One non-carbon group is replaced by another group. Usually only occurs at a singly bonded site. The only example so far is halogen/UV on alkanes but alkanols and haloalkanes love a bit of substitution as well. SE – electrophylic substitution – электрофильное замещение SN – nucleophylic substitution – нуклеофильное замещение 4. Condensation and Hydrolysis Condensation is where two molecules join together and lose a simple molecule. e.g. water or carbon dioxide. Hydrolysis is the reverse. 5. Oxidation and Reduction Are opposite of each other. Oxidation is either adding O or making it have a double bond or removing H or both. Reduction is the opposite. 6. Isomerization This is when a substance changes into another form with different properties but the same molecular formula. For example: n -pentane → iso -pentane The different forms are called “isomers”, and the phenomenon of the existence of different forms “isomerism”. Polymerization This is when a substance changes into another substance with the same composition but a much higher molecular mass. For example: nC2H4ethylene (monomer)→ (–CH2–CH2–)n polyethylene (polymer)) The product of such a reaction is called a “polymer”, and the starting material the corresponding “monomer”. The term polymerization is also used for processes in which a polymer is formed, not from the monomer, but from other reactants of low molecular mass. This usage is somewhat misleading, but is well established.
Oligomerization This is similar to polymerization except that the product contains only a small number of monomer units. For example: 3C2H2 —heat, catalyst→ C6H6The degree of oligomerization is specified by the numerical prefixes di-, tri-, etc., as in dimer, trimerize, etc.
Substitution Reactions of Benzene Because of its greater stability than alkenes benzene tends to undergo substitution reactions rather than addition reactions (as do the alkenes). Nitration. Benzene reacts with a mixture of concentrated nitric and sulfuric acids to produce nitrobenzene as shown in the reaction below. The process is termed nitration. Sulfuric acid is the catalyst and the mixture must be warmed to about 500оC to initiate the reaction Halogenation. Both bromine and chlorine react with benzene if iron is present. The actual catalyst is FeCl3 or FeBr3 (metallic iron also works as a catalyst because it reacts with halogens to produce the above salts). Alkylation (Friedel-Crafts reaction) Substitution of an alkyl group onto a benzene ring is called alkylation. The reaction is very similar to halogenations except that aluminum chloride is used to catalyze the reaction, as shown in the reaction below. In general alkyl halide RX is used in the substitution to produce an alkyl-benzene.
Aromatic hydrocarbons. Benzene and derivatives of benzene. These are compounds that contain the benzene ring as part of their structure.Benzene is used mainly as an intermediate to make other chemicals. About 80% of benzene is consumed in the production of three chemicals, ethylbenzene, cumene, and cyclohexane. Its most widely produced derivative is ethylbenzene, precursor to styrene, which is used to make polymers and plastics. In laboratory research, toluene is now often used as a substitute for benzene. The solvent-properties of the two are similar, but toluene is less toxic and has a wider liquid range. Benzene represents a special problem in that, to account for the bond lengths quantitatively, there must either be electron delocalization (molecular orbital theory) or a spin coupling of the p-orbitals (valence bond theory)
Nomenclature of Aromatic Compounds For molecules having alkyl groups or other simple substituents attached to the benzene ring benzene is used as the parent name and the attached groups are named in alphabetical order before it. If more than one substituent is attached to the benzene ring, their locations are specified by numbers. The lowest possible numbers are used and the substituents are named in alphabetical order. 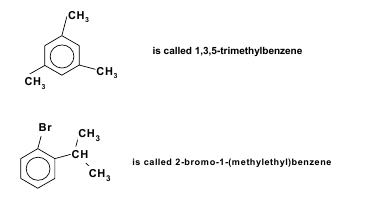 When only two substituents are present the prefixes ortho-, meta- and para are used in place of 1, 2-, 1, 3- and 1, 4- respectively.
When only two substituents are present the prefixes ortho-, meta- and para are used in place of 1, 2-, 1, 3- and 1, 4- respectively. 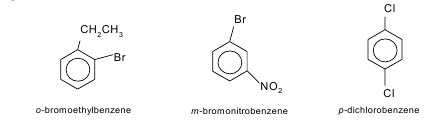 Ortho-, meta- and para- are generally abbreviated to o-, m- and p- in most cases. Benzene derivatives have from one to six substituents attached to the central benzene core. Examples of benzene compounds with just one substituent are phenol, which carries a hydroxyl group, and toluene with a methyl group. When there is more than one substituent present on the ring, their spatial relationship becomes important for which the arene substitution patterns ortho, meta, and para are devised. For example, three isomers exist for cresol because the methyl group and the hydroxyl group can be placed next to each other (ortho), one position removed from each other (meta), or two positions removed from each other (para). Xylenol has two methyl groups in addition to the hydroxyl group, and, for this structure, 6 isomers exist
Ortho-, meta- and para- are generally abbreviated to o-, m- and p- in most cases. Benzene derivatives have from one to six substituents attached to the central benzene core. Examples of benzene compounds with just one substituent are phenol, which carries a hydroxyl group, and toluene with a methyl group. When there is more than one substituent present on the ring, their spatial relationship becomes important for which the arene substitution patterns ortho, meta, and para are devised. For example, three isomers exist for cresol because the methyl group and the hydroxyl group can be placed next to each other (ortho), one position removed from each other (meta), or two positions removed from each other (para). Xylenol has two methyl groups in addition to the hydroxyl group, and, for this structure, 6 isomers exist
Heterocyclic and polyaromatic hydrocarbons. Polyaromatic hydrocarbons. Polyaromatic hydrocarbons (PAH s), also known as polycyclic aromatic hydrocarbons or polynuclear aromatic hydrocarbons are potent atmospheric pollutants that consist of fused aromatic rings and do not contain heteroatoms or carry substituents. Naphthalene is the simplest example of a PAH. PAHs occur in oil, coal, and tar deposits, and are produced as byproducts of fuel burning (whether fossil fuel or biomass). As a pollutant, they are of concern because some compounds have been identified as carcinogenic, mutagenic, and teratogenic. PAHs are also found in cooked foods. Studies have shown that high levels of PAHs are found, for example, in meat cooked at high temperatures such as grilling or barbecuing, and in smoked fish.
Fundamentals of biochemistry. Types of biomolecules. Biochemistry, sometimes called biological chemistry, is the study of chemical processes within and relating to, living organisms. By controlling information flow through biochemical signaling and the flow of chemical energy through metabolism, biochemical processes give rise to the complexity of life. Over the last 40 years, biochemistry has become so successful at explaining living processes that now almost all areas of the life sciences from botany to medicine are engaged in biochemical research. Today, the main focus of pure biochemistry is in understanding how biological molecules give rise to the processes that occur within living cells, which in turn relates greatly to the study and understanding of whole organisms.Proteins
Белки) Proteins are large biological molecules, or macromolecules, consisting of one or more chains of amino acid residues.Lipids(Липиды) Lipids are a group of naturally occurring molecules that include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E, and K), monoglycerides, diglycerides, triglycerides, phospholipids, and others.Carbohydrates(Углеводы) A carbohydrate is a large biological molecule, or macromolecule, consisting only of carbon (C), hydrogen (H), and oxygen (O), usually with a hydrogen: oxygen atom ratio of 2: 1 (as in water); in other words, with the empirical formula Cm(H2O)n (where m could be different from n).Nucleic acids(Нуклеиновые кислоты) Nucleic acids are polymeric macromolecules, or large biological molecules, essential for all known forms of life. Nucleic acids, which include DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), are made from monomers known as nucleotides.Vitamines(Витамины) A vitamin is an organic compound required by an organism as a vital nutrient in limited amounts. An organic chemical compound (or related set of compounds) is called a vitamin when it cannot be synthesized in sufficient quantities by an organism, and must be obtained from the diet. Thus, the term is conditional both on the circumstances and on the particular organismEnzymes(Ферменты) Enzymes are large biological molecules responsible for the thousands of metabolic processes that sustain life. They are highly selective catalysts, greatly accelerating both the rate and specificity of metabolic reactions, from the digestion of food to the synthesis of DNA. Most enzymes are proteins.
Carbohydrates. Carbohydrate Classification There are a variety of interrelated classification schemes. The most useful classification scheme divides the carbohydrates into groups according to the number of individual simple sugar units. Monosaccharides contain a single unit; disaccharides contain two sugar units; and polysaccharides contain many sugar units as in polymers - most contain glucose as the monosaccharide unit. Monosaccharides: Glucose, Galactose, Fructose, Ribose, Glyceraldehyde. Disaccharides: Sucrose, Maltose, Lactose Polysaccharides: Starch, Glycogen, Cellulose. Functional Groups Aldoses contain the aldehyde group - Monosaccharides in this group are glucose, galactose, ribose, and glyceraldehyde. Ketoses contain the ketone group - The major sugar in this group is fructose. Reducing: Contain a hemiacetal or hemiketal group. Sugars include, glucose, galactose, fructose, maltose, lactose Non-reducing: Contain no hemiacetal groups. Sucrose and all polysaccharides are in this group. Monosaccharides: Monosaccharides are formed by a single molecule. It means that when hydrolyzed they can not release simpler molecules. Examples of this group of carbohydrates are glucose, ribose and fructose, among others. Oligosaccharides. They are formed by 2-9 monomers linked through glycosidic linkages; in other words, when hydrolyzed these compounds release 2 to 9 monosaccharides (some texts say up to 20; in fact, oligosaccharides release “a few” monosaccharides). Polysaccharides: Polysaccharides are carbohydrates formed by more than 9 monosaccharides (some texts say more than 10 monosaccharides, other texts say more than 20…in fact, they usually are formed by a lot of monosaccharides!). When the polysaccharides are formed by the same type of monosaccharides, they are called homopolysaccharides.
Amino acids. Proteins and its structure. Enzymes. Amino acids are biologically important organic compounds composed of amine (-NH2) and carboxylic acid (-COOH) functional groups, along with a side-chain specific to each amino acid. The key elements of an amino acid are carbon, hydrogen, oxygen, and nitrogen, though other elements are found in the side-chains of certain amino acids. Proteinogenic amino acids are amino acids that are precursors to proteins, and are produced by cellular machinery coded for in the genetic code of any organism. There are 22 standard amino acids, but only 21 are found in eukaryotes. Of the 22, selenocysteine and pyrrolysine are incorporated into proteins by distinctive biosynthetic mechanisms. The other 20 are directly encoded by the universal genetic code. Humans can synthesize 11 of these 20 from each other or from other molecules of intermediary metabolism. The other 9 must be consumed (usually as their protein derivatives) in the diet and so are thus called essential amino acids. The essential amino acids are histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine Protein structure is the biomolecular structure of a protein molecule. Each protein is a polymer – specifically a polypeptide – that is a sequence formed from various L-α -amino acids (also referred to as residues) vitamins. A vitamin is an organic compound and a vital nutrient that an organism requires in limited amounts. An organic chemical compound is called a vitamin when the organism cannot synthesize the compound in sufficient quantities, and must be obtained through the diet; thus, the term " vitamin" is conditional upon the circumstances and the particular organism. For example, ascorbic acid (vitamin C) is a vitamin for humans, but not for most other animal organisms.Vitamin A (Retinol)- Cod liver oil; Vitamin B1 (Thiamine)-Rice bran; Vitamin C (Ascorbic acid)-Citrus, most fresh foods; Vitamin D (Calciferol)- Cod liver oil; Vitamin E) (Tocopherol)- Wheat germ oil, unrefined vegetable oils; Vitamin B2 (Riboflavin)-Meat, dairy products, eggs. Vitamins are classified as either water-soluble or fat-soluble. In humans there are 13 vitamins: 4 fat-soluble (A, D, E, and K) and 9 water-soluble (8 B vitamins and vitamin C).
Аlkaloids and hormones. Alkaloids are a group of naturally occurring chemical compounds, that contain mostly basic nitrogen atoms. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure are also attributed to alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and more rarely other elements such as chlorine, bromine, and phosphorus.Contains nitrogen - usually derived from an amino acid. Bitter tasting, generally white solids (exception - nicotine is a brown liquid). They give a precipitate with heavy metal iodides: Most alkaloids are precipitated from neutral or slightly acidic solution by Mayer's reagent (potassiomercuric iodide solution). Cream coloured precipitate. Dragendorff's reagent (solution of potassium bismuth iodide) gives orange coloured precipitate with alkaloids. Caffeine, a purine derivative, does not precipitate like most alkaloids. Alkaloids are basic - they form water soluble salts. Most alkaloids are well-defined crystalline substances which unite with acids to form salts. In plants, they may exist in the free state, as salts or as N-oxides. Occur in a limited number of plants. Nucleic acid exists in all plants, whereas, morphine exists in only one plant species.Alkaloids can be classified; in terms of their BIOLOGICAL activity, CHEMICAL structure (nucleus containing nitrogen), BIOSYNTHETIC pathway (the way they are produced in the plant). HORMONES A hormone is a class of regulatory biochemicals produced in particular parts of organisms by specific cells, glands, and/or tissues and then transported by the bloodstream to other parts of the body, with the intent of influencing a variety of physiological and behavioral activities, such as the processes of digestion, metabolism, growth, reproduction, and mood control. Hormonal signaling involves the following: Biosynthesis of a particular hormone in a particular tissue. Storage and secretion of the hormone. Transport of the hormone to the target cell(s). Recognition of the hormone by an associated cell membrane or intracellular receptor protein. Relay and amplification of the received hormonal signal via a signal transduction process: This then leads to a cellular response. The reaction of the target cells may then be recognized by the original hormone-producing cells, leading to a down-regulation in hormone production. This is an example of a homeostatic negative feedback loop. Those classes of hormones are found too in other groups of animals. In insects and crustaceans, there is a hormone with an unusual chemical structure, compared with other animal hormones, the juvenile hormone, a sesquiterpenoid
Nucleic acids. RNA. DNA. Nucleic acids are polymolecules, or large biomolecules, essential for all known forms of life. Nucleic acids, which include DNA (deoxyribonucleic acid) and RNA (ribonucleic acid), are made from monomers known as nucleotides. Each nucleotide has three components: a 5-carbon sugar, a phosphate group, and a nitrogenous base. If the sugar is deoxyribose, the polymer is DNA. If the sugar is ribose, the polymer is RNA.Nucleic acids were discovered by Friedrich Miescher in 1869. Deoxyribonucleic acid (DNA) is a nucleic acid containing the genetic instructions used in the development and functioning of all known living organisms (with the exception of RNA viruses).Ribonucleic acid (RNA) functions in converting genetic information from genes into the amino acid sequences of proteins. The three universal types of RNA include transfer RNA (tRNA), messenger RNA (mRNA), and ribosomal RNA (rRNA).
Analytical chemistry is one of the chemical disciplines. Analytical chemistry is united with other chemical sciences with common chemical laws and based on studying of chemical properties of substances. Analytical chemistry is the study of the separation, identification, and quantification of the chemical components of natural and artificial materials. Qualitative analysis gives an indication of the identity of the chemical species in the sample, and quantitative analysis determines the amount of certain components in the substance. The separation of components is often performed prior to analysis. Analytical chemistry is the chemical science about: – theoretical base of chemical analysis of substances; – method of detection and identification of chemical elements; – methods of qualitative determination of substances; – methods of selection (separation) of chemical elements and its compounds; – methods of establishing the structure of chemical compounds. Subjects of analytical chemistry are: chemical elements and its compounds and processing of transformation of substances in run chemical reactions. Aims of analytical chemistry are: 1. Establishing the chemical composition of analyzed object (isotopic, elementary, ionic, molecular, phase) – qualitative analysis. Qualitative analysis consist from – identification – establishing of identity of researched chemical compounds with well-known substance due to compare its physical and chemical properties – and detection – checking the presence in analyzed objects some components, impurities, functional groups etc. 2. Determination of content (amount and concentration) some components in analyzed objects – quantitative analysis. 3. Determination (establishing) of structure of chemical compound – nature and number of structural elements, its bonds one to another, disposition in space. 4. Detection of heterogeneity on surface or in volume of solids, distribution of elements in layers. 5. Research process in time: establishing character, mechanism and rate of molecular regrouping.6. Developing of present analytical methods theory, working out the new methods of analysis.
The role of analytical chemistry. Analytical chemistry is applied throughout industry, medicine, and all the sciences. To illustrate, consider a few examples. The concentrations of oxygen and of carbon dioxide are determined in millions of blood samples every day and used to diagnose and treat illnesses. Quantities of hydrocarbons, nitrogen oxides, and carbon monoxide present in automobile exhaust gases are measured to determine the effectiveness of emission-control devices. Quantitative measurements of ionized calcium in blood serum help diagnose parathyroid disease in humans. Quantitative determination of nitrogen in foods establishes their protein content and thus their nutritional value. The interdisciplinary nature of chemical analysis makes it a vital tool in medical, industrial, government, and academic laboratories throughout the world.
Analytical chemistry achieves the aims by various methods of analysis: I. Physical – determination of components of investigated substances without chemical reactions (destroying of sample): 1.Spectral analysis – investigation of emission and absorption spectra.2. Fluorescence analysis – investigation of luminescence, caused action of UV-radiation. 3. Roentgen-structural analysis – using X-ray.4.Mass-spectra analysis.5.Densimetry – measurement of density. II. Instrumental (physical-chemical) – based on measurement of physical parameters (properties) of substances in run of chemical reaction. This method divides on 1.Electrochemical– measurement of electrical parameters of electrochemical reactions. 2. Optical – investigation the influence of various electromagnetic radiation on substance.3.Thermal (heating) – investigation the changes the properties of substance by heat (undergo) action. III. Chemical – measurement of chemical bonds energy.Chemical analysis has some steps: 1. Sampling. 2. Dissolving the sample (in water, acid or alkali).3. Executing (running) the chemical reaction X + R → P.4. Measurement of definite parameter. In accordance to analytical reaction (X + R → P) applies three groups of chemical analysis methods: Measurement of amount (quantity) of reaction product P: mass, physical properties. Measurement of amount of reagent R that interacted with determined substance: volume of solution reagent R with known concentration. Registration changes of substance X acting with reagent: measurement of gas volumes.
Chemical methods of analysis. Titration. Titration, also known as titrimetry, is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte. Since volume measurements play a key role in titration, it is also known as volumetric analysis. A reagent, called the titrant or titrator is prepared as a standard solution. A known concentration and volume of titrant reacts with a solution of analyte or titrand to determine concentration Procedure A typical titration begins with a beaker or Erlenmeyer flask containing a very precise volume of the analyte and a small amount of indicator placed underneath a calibrated burette or chemistry pipetting syringe containing the titrant. Preparation techniques Typical titrations require titrant and analyte to be in a liquid (solution) form. Though solids are usually dissolved into an aqueous solution, other solvents such as glacial acetic acid or ethanol are used for special purposes (as in petrochemistry). Concentrated analytes are often diluted to improve accuracy. Types of titrations There are many types of titrations with different procedures and goals. The most common types of qualitative titration are acid-base titrations and redox titrations. Acid–base titration Acid-base titrations depend on the neutralization between an acid and a base when mixed in solution. In addition to the sample, an appropriate indicator is added to the titration chamber, reflecting the pH range of the equivalence point. Redox titration Redox titrations are based on a reduction-oxidation reaction between an oxidizing agent and a reducing agent. A potentiometer or a redox indicator is usually used to determine the endpoint of the titration, as when one of the constituents is the oxidizing agent potassium dichromate. Complexometric titration Complexometric titrations rely on the formation of a complex between the analyte and the titrant. In general, they require specialized indicators that form weak complexes with the analyte. Complexometric titration Complexometric titrations rely on the formation of a complex between the analyte and the titrant. In general, they require specialized indicators that form weak complexes with the analyte. Endpoint and equivalence point Though equivalence point and endpoint are used interchangeably, they are different terms. Equivalence point is the theoretical completion of the reaction: the volume of added titrant at which the number of moles of titrant is equal to the number of moles of analyte, or some multiple thereof (as in polyprotic acids). Back titration Back titration is a titration done in reverse; instead of titrating the original sample, a known excess of standard reagent is added to the solution, and the excess is titrated.
Instrumental analysis- Instrumental analysis is a field of analytical chemistry that investigates analytes using scientific instruments. Spectroscopy is the study of the interaction between matter and radiated energy. Mass spectrometry (MS) is an analytical technique that produces spectra (singular spectrum) of the masses of the atoms or molecules comprising a sample of material. Electroanalytical methods are a class of techniques in analytical chemistry which study an analyte by measuring the potential (volts) and/or current (amperes) in an electrochemical cell containing the analyte. Potentiometry Potentiometry passively measures the potential of a solution between two electrodes, affecting the solution very little in the process. The potential is then related to the concentration of one or more analytes. Coulometry Coulometry uses applied current or potential to completely convert an analyte from one oxidation state to another. In these experiments, the total current passed is measured directly or indirectly to determine the number of electrons passed Voltammetry Voltammetry applies a constant and/or varying potential at an electrode's surface and measures the resulting current with a three electrode system. This method can reveal the reduction potential of an analyte and its electrochemical reactivity. Polarography Polarography is a subclass of voltammetry that uses a dropping mercury electrode as the working electrode. Amperometry Amperometry is the term indicating the whole of electrochemical techniques in which a current is measured as a function of an independent variable that is, typically, time or electrode potential.
Chromatography is the collective term for a set of laboratory techniques for the separation of mixtures. The mixture is dissolved in a fluid called the mobile phase, which carries it through a structure holding another material called the stationary phase. The various constituents of the mixture travel at different speeds, causing them to separate. The separation is based on differential partitioning between the mobile and stationary phases. Chromatography was first employed by Russian scientist Mikhail Tsvet in 1900. Column chromatography Column chromatography is a separation technique in which the stationary bed is within a tube. Planar chromatography is a separation technique in which the stationary phase is present as or on a plane. The plane can be a paper, serving as such or impregnated by a substance as the stationary bed (paper chromatography) or a layer of solid particles spread on a support such as a glass plate (thin layer chromatography). Paper chromatography Paper chromatography is a technique that involves placing a small dot or line of sample solution onto a strip of chromatography paper. The paper is placed in a jar containing a shallow layer of solvent and sealed. Thin layer chromatography (TLC) is a widely employed laboratory technique and is similar to paper chromatography. However, instead of using a stationary phase of paper, it involves a stationary phase of a thin layer of adsorbent like silica gel, alumina, or cellulose on a flat, inert substrate. Gas chromatography (GC), also sometimes known as gas-liquid chromatography, (GLC), is a separation technique in which the mobile phase is a gas. Gas chromatographic separation is always carried out in a column, which is typically " packed" or " capillary". Liquid chromatography Liquid chromatography (LC) is a separation technique in which the mobile phase is a liquid. Liquid chromatography can be carried out either in a column or a plane Supercritical fluid chromatography Supercritical fluid chromatography is a separation technique in which the mobile phase is a fluid above and relatively close to its critical temperature and pressure. Ion exchange chromatography Ion exchange chromatography (usually referred to as ion chromatography) uses an ion exchange mechanism to separate analytes based on their respective charges.
Law of mass action (LMA). K.Guldberg and P.Waage (1867) clearly stated the Law of Mass Action (sometimes termed the Law of Chemical Equilibrium) in the form: the rate of any chemical reaction is proportional to the product of the masses of the reacting substances, with each mass raised to a power equal to the coefficient that occurs in the chemical equation. “Active mass” was interpreted as concentration and expressed in moles per liter. The law of mass action is a reaction that states that the values of the equilibrium – constant expression Kc are constant for a particular reaction at a given temperature, whatever equilibrium concentrations are substitute
Application of the law of mass action- Chemically pure water conducts an electric current very poorly and has an electrical conductivity of 0.055 µS∙ cm− 1. But nevertheless it has a measurable electrical conductivity that is explained by the slight dissociation of water into hydrogen and hydroxide ions (According to the theories of Svante Arrhenius): H2O → H+ + OH– The electrical conductivity of pure water can be used to calculate the concentration of hydrogen and hydroxide ions in water. Let us write an expression for the dissociation constant of water:  We can rewrite this equation as follows: [H+]∙ [OH–] = [H2O]∙ K Replacing the product [H2O]∙ K in the last equation with the new constant Kw, we have: [H+]∙ [OH–] = KwThe latter Kw is called the ion product of water (or ionization constant, dissociation constant, self-ionization constant). The Kw value is depended of temperature. For pure water at 25°C Kw=10-14, we have [H+] = [OH-] = 1·10-7 mol/L. Hence, for this temperature: Kw = 10-7 ∙ 10-7 = 10-14 Solutions in which the concentrations of the hydrogen ions and hydroxide ions are the same are called neutral solutions.
We can rewrite this equation as follows: [H+]∙ [OH–] = [H2O]∙ K Replacing the product [H2O]∙ K in the last equation with the new constant Kw, we have: [H+]∙ [OH–] = KwThe latter Kw is called the ion product of water (or ionization constant, dissociation constant, self-ionization constant). The Kw value is depended of temperature. For pure water at 25°C Kw=10-14, we have [H+] = [OH-] = 1·10-7 mol/L. Hence, for this temperature: Kw = 10-7 ∙ 10-7 = 10-14 Solutions in which the concentrations of the hydrogen ions and hydroxide ions are the same are called neutral solutions.
pH scale. The pH of pure water In fact, pure water only has a pH of 7 at a particular temperature 25oC - the temperature at which the Kw value is 1.00 x 10-14 mol2 dm-6. . This is how it comes about: To find the pH you need first to find the hydrogen ion concentration (or hydroxonium ion concentration - it's the same thing). Then you convert it to pH In pure water at room temperature the Kw value tells you that: [H+] [OH-] = 1.00 · 10-14 But in pure water, the hydrogen ion (hydroxonium ion) concentration must be equal to the hydroxide ion concentration. For every hydrogen ion formed, there is a hydroxide ion formed as well. That means that you can replace the [OH-] term in the Kw expression by another [H+].H+]2 = 1.00 x 10-14 Taking the square root of each side gives: [H+] = 1.00 · 10-7 mol dm-3 Converting that into pH: pH = - lg[H+] pH = 7 That's where the familiar value of 7 comes from. pH and pOH Because the constant of water, Kw is always 1.0·10-14, the pKw is 14, the constant of water determines the range of the pH scale. To understand what the pKw is, it is important to understand first what the " p" means in pOH, and pH.
Electrolytes and non-electrolytes. Contemporary Theories of Electrolytes A substance that dissolves in water to give an electrically conducting solution is called an electrolyte. A substance that dissolves in water to give no conducting or very poorly conducting solutions is called a nonelectrolyte. According to Svante Arrhenius concept: Acid is any substance that, when dissolved in water, increase the concentration of hydrogen ion H+. Base is any substance that, when dissolved in water, increase the concentration of hydroxide ion OH–. NaOH → Na+ + OH– HCl → H+ + Cl– According to Johannes N. Brö nsted and Thomas M. Lowry concept: Acid is the species (molecule or ion) that donates a proton to another species in a proton-transfer reaction. Base is the species (molecule or ion) that accepts a proton in a proton-transfer reaction. HCl + NH3 → NH4Cl NH3 + H2O → NH4+ + OH– According to G.N.Lewis concept: Lewis acid is a species that can form a covalent bond by accepting an electron pair from another species. Lewis base is a species that can form a covalent bond by donating an electron pair to another species. Acids and bases are classified as strong or weak. Strong acids are acids that ionize completely in water (that is, they react completely to give ions). Weak acids are acids that are only partly ionized as the result of equilibrium reaction with water. Strong bases are bases that are present in aqueous solution entirely as ions, one of which is OH–. Weak bases are bases that are only partly ionized as the result of equilibrium reactions with water. Acid and base with water produce hydrogen ion or hydroxide ion (relatively) and its conjugated ions. The process is called electrolyte ionization or electrolyte dissociation.
Properties of Ionic Compounds in Aqueous Solution The properties of ionic compounds in solution are actually the properties of the individual ions themselves. These compounds are called strong electrolytes because their solutions conduct electricity well. For example, an aqueous solution of sodium chloride consists essentially of sodium ions and chloride ions in water. Strong acids, strong bases, and salts all provide ions in solution. They are all strong electrolytes, but the process by which these types of compounds form ions in solution differs. When they are pure, strong acids are covalent compounds, but they undergo a chemical reaction with water to form ions in solution. Salts and strong bases are ionic even when they are pure, and their interaction with water is more a physical process than a chemical reaction. Weak acids and bases ionize only slightly in aqueous solution. Because their solutions conduct electricity poorly, they are called weak electrolytes. Compounds whose solutions do not conduct electricity at all are called non-electrolytes. Writing Net Ionic Equations We will fi nd net ionic equations extremely useful for summarizing a great deal of information with relatively little effort. Three Types of Equations Are Used to Describe Reactions in Solution: 1. The formula (total) equation gives the overall reaction stoichiometry but not necessarily the actual forms of the reactants and products in solution. 2. The complete ionic equation represents as ions all reactants and products that are strong electrolytes. 3. The net ionic equation includes only those solution components undergoing a change. Spectator ions are not included.
Colloidal Chemistry. Types of disperse systems. Materials may be mixed together to form a true solution, a coloidal dispersion, or a coarse dispersion. A true solution - mixture of two or more components that form a homogenous molecular dispersion, i.e. a one-phase system, the composition of which can vary over a wide range. A colloidal dispersion - represents a system havin a particle size intermediate between that of a true solution and a coarse dispersion, roughly 10Å to 5000Å (0.1mm = 1000Å) A coarse dispersion the diameter of the particles in emulsions and suspensions for the most part being larger than 0.1mm (1000Å).. In order to be distributed in this way, the colloidal mixture has to be broken down into very small particles, called colloidal particles, which are too small to be directly seen by a conventional microscope. A solution is a homogeneous mixture of one or more solutes dissolved in a solvent. solvent: the substance in which a solute dissolves to produce a homogeneous mixture solute: the substance that dissolves in a solvent to produce a homogeneous mixture. Many different kinds of solutions exist. For example, a solute can be a gas, a liquid or a solid. Solvents can also be gases, liquids or solids.Colloids are on the dividing line between solutions and heterogeneous mixtures. Like solutions, colloids can be gases, liquids, or solids.
Thermodynamics. Enthalpy. Spontaneous Processes and Entropy.Free Gibbs energy. Energy added to a system is defi ned as positive, and energy released from a system is defi ned as negative. A process in which energy is added to a system is said to be an endothermic process. A process in which energy is released from a system is said to be an exothermic process. The study of energy and its interconversions is called thermodynamics. The law of conservation of energy is often called the fi rst law of thermodynamics and is stated as follows: The energy of the universe is constant. Thermodynamics Thermodynamic parameters on changes of state are necessary to describe chemical bonding, structure, and reaction. Enthalpy Since enthalpy is the heat content of a system under constant pressure, the change ∆ H is negative in an exothermic reaction, and positive in an endothermic reaction. Spontaneous Processes and Entropy Thermodynamics lets us predict whether a process will occur but gives no information about the amount of time required for the process. For example, according to the principles of thermodynamics, a diamond should change spontaneously to graphite. Free Gibbs energy So far we have used to predict the spontaneity of a process. However, another thermodynamic function is also related to spontaneity and is especially useful in dealing with the temperature dependence of spontaneity. This function is called the free energy, which is symbolized by G and defi ned by the relationship Δ G = Δ H – TΔ S
Enthalpy of Formation One particularly useful type of process for us to consider is the formation of a substance in its standard state from its elements in their standard states. Standard state is the state in which the substance is most stable. For example, the standard state of elemental oxygen is O2(g) not O(g) or O3(g). Hess’s Law Since enthalpy is a state function, the change in enthalpy in going from some initial state to some fi nal state is independent of the pathway. This means that in going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps. To use Hess’s law to compute enthalpy changes for reactions, it is important to understand two characteristics of Δ H for a reaction: 1. If a reaction is reversed, the sign of Δ H is also reversed. 2. The magnitude of Δ H is directly proportional to the quantities of reactants and products in a reaction. If the coeffi cients in a balanced reaction are multiplied by an integer, the value of Δ H is multiplied by the same integer
Hydrogen is a chemical element with chemical symbol H and atomic number 1. With an atomic weight of 1.00794 u, hydrogen is the lightest element on the periodic table. The universal emergence of atomic hydrogen first occurred during the recombination epoch. At standard temperature and pressure, hydrogen is a colorless, odorless, tasteless, non-toxic, nonmetallic, highly combustible diatomic gas with the molecular formula H2. Since hydrogen readily forms covalent compounds with most non-metallic elements, most of the hydrogen on Earth exists inmolecular forms such as in the form of water or organic compounds.I
Oxygen is a chemical element with symbol O and atomic number 8. It is a member of the chalcogen group on the periodic tableand is a highly reactive nonmetallic element and oxidizing agent that readily forms compounds (notably oxides) with most elements.[3] By mass, oxygen is the third-most abundant element in the universe, after hydrogen and helium.[4] At STP, two atoms of the element bind to form dioxygen, a diatomic gas that is colorless, odorless, and tasteless, with the formula O Many major classes of organic molecules in living organisms, such as proteins, nucleic acids, carbohydrates, and fats, contain oxygen, as do the major inorganic compounds that are constituents of animal shells, teeth, and bone.
Carbon (from Latin: carbo " coal") is a chemical element with symbol C and atomic number 6. As a member of group 14 on the periodic table, it is nonmetallic and tetravalent—making four electrons available to form covalent chemical bonds. There are three naturally occurring isotopes, with 12C and 13C being stable, while 14C is radioactive, decaying with a half-life of about 5, 730 years.[14] Carbon is one of the few elements known since antiquityThere are several allotropes of carbon of which the best known are graphite, diamond, and amorphous carbon.[16] Thephysical properties of carbon vary widely with the allotropic form.
Nitrogen is a chemical element with symbol N and atomic number 7. It is the lightest pnictogen and at room temperature, it is a transparent, odorless diatomic gas. Nitrogen is a common element in the universe, estimated at about seventh in total abundance in the Milky Way and the Solar System. On Earth, the element forms about 78% of Earth's atmosphere and as such is the most abundant uncombined element. The element nitrogen was discovered as a separable component of air, by Scottish physicianDaniel Rutherford, in 1772.Many industrially important compounds, such as ammonia, nitric acid, organic nitrates (propellants and explosives), and cyanides, contain nitrogen.
Sodium /ˈ soʊ diə m/[4] is a chemical element with symbol Na (from Latin: natrium) and atomic number 11. It is a soft, silver-white, highly reactive metal and is a member of the alkali metals; its only stable isotope is 23Na. The free metal does not occur in nature, but instead must be prepared from its compounds. It was first isolated by Humphry Davy in 1807 by the electrolysisof sodium hydroxide. Sodium is the sixth most abundant element in the Earth's crust, and exists in numerous minerals such asfeldspars, sodalite and rock salt (NaCl). Many salts of sodium are highly water-soluble Many sodium compounds are useful, such as sodium hydroxide (lye) for soap-making, and sodium chloride for use as a de-icing agent and a nutrient (edible salt).
Aluminium (or aluminum; see spelling differences) is a chemical element in the boron group with symbol Al and atomic number 13. It is a silvery-white, soft, nonmagnetic, ductile metal. Aluminium is the third most abundant element (after oxygen andsilicon), and the most abundant metal in the Earth's crustAluminium is remarkable for the metal's low density and for its ability to resist corrosion due to the phenomenon of passivation. Structural components made from aluminium and its alloys are vital to the aerospace industry and are important in other areas oftransportation and structural materials. The most useful compounds of aluminium, at least on a weight basis, are the oxides andsulfates.
Silicon is a chemical element with symbol Si and atomic number 14. It is a tetravalent metalloid, more reactive than germanium, the metalloid directly below it in the table. Controversy about silicon's character dates to its discovery; it was first prepared and characterized in pure form in 1823. In 1808, it was given the name silicium (from Latin: silex, hard stone or flint), with an -ium word-ending to suggest a metal, a name which the element retains in several non-English languages. However, its final English name, first suggested in 1817, reflects the more physically similar elements carbon and boron.Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure free element in nature. It is most widely distributed in dusts, sands, planetoids, and planets as various forms of silicon dioxide (silica) or silicates. Over 90% of the Earth's crust is composed of silicate minerals, making silicon the second most abundant element in the Earth's crust (about 28% by mass) after oxygen.[9]
Most silicon is used commercially without being separated, and indeed often with little processing of compounds from nature. These include direct industrial building-use of clays, silica sand and stone. Silicate goes into Portland cement for mortar andstucco, and when combined with silica sand and gravel, to make concrete. Silicates are also in whiteware ceramics such asporcelain, and in traditional quartz-based soda-lime glass and many other specialty glasses. More modern silicon compounds such as silicon carbide form abrasives and high-strength ceramics. Silicon is the basis of the widely used synthetic polymers called silicones.
Phosphorus is a chemical element with symbol P and atomic number 15. As an element, phosphorus exists in two major forms—white phosphorus and red phosphorus—but due to its high reactivity, phosphorus is never found as a free element on Earth. Instead phosphorus-containing minerals are almost always present in their maximally oxidised state, as inorganic phosphate rocks. Phosphorus is essential for life. Phosphates (compounds containing the phosphate ion, PO4− 3) are a component of DNA, RNA, ATP, and also the phospholipids, which form all cell membranes.
Sulfur or sulphur (see spelling differences) is a chemical element with symbol S and atomic number 16. It is an abundant, multivalent non-metal. Under normal conditions, sulfur atoms form cyclic octatomic molecules with chemical formula S8. Elemental sulfur is a bright yellow crystalline solid when at room temperature. Chemically, sulfur can react as either an oxidant or a reducing agent. It oxidizes most metals and several nonmetals, including carbon, which leads to its negative charge in most organosulfur compounds, but it reduces several strong oxidants, such as oxygen and fluorine.Sulfur occurs naturally as the pure element (native sulfur) and as sulfide and sulfate minerals. Elemental sulfur crystals are commonly sought after by mineral collectors for their distinct, brightly colored polyhedron shapes.
Fluorine is a chemical element with symbol F and atomic number 9. It is the lightest halogen and exists as a highly toxic pale yellow diatomic gas at standard conditions. As the most electronegative element, it is extremely reactive: almost all other elements, including some noble gases, form compounds with fluorine.
Among the elements, fluorine ranks 24th in universal abundance and 13th in terrestrial abundance. Fluorite, the primary mineral source of fluorine, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb fluo meaning " flow" became associated with it.
Chlorine is a chemical element with symbol Cl and atomic number 17. Chlorine is in the halogen group (17) and is the second lightest halogen following fluorine. The element is a yellow-green gas under standard conditions, where it forms diatomic molecules. Chlorine has the highest electron affinity and the third highest electronegativity of all the reactive elements. For this reason, chlorine is a strong oxidizing agent. Free chlorine is rare on Earth, and is usually a result of direct or indirect oxidation byoxygen.The most common compound of chlorine, sodium chloride (common salt), has been known since ancient times
Bromine (from Greek: β ρ ῶ μ ο ς, bró mos, meaning " strong-smelling" or " stench")[5] is a chemical element with symbol Br, andatomic number 35. It is a halogen. The element was isolated independently by two chemists, Carl Jacob Lö wig and Antoine Jerome Balard, in 1825–1826. Elemental bromine is a fuming red-brown liquid at room temperature, corrosive and toxic, with properties between those of chlorine and iodine. Free bromine does not occur in nature, but occurs as colorless soluble crystalline mineral halide salts, analogous to table salt.
Iodine is a chemical element with symbol I and atomic number 53. The name is from Greek ἰ ο ε ι δ ή ς ioeidē s, meaning violet or purple, due to the color of elemental iodine vapor.[3]
Iodine and its compounds are primarily used in nutrition, and industrially in the production of acetic acid and certain polymers. Iodine's relatively high atomic number, low toxicity, and ease of attachment to organic compounds have made it a part of many X-ray contrast materials in modern medicine. Iodine has only one stable isotope. A number of iodine radioisotopes, such as 131I, are also used in medical applications.
Potassium is a chemical element with the symbol K (derived from Neo-Latin kalium) and atomic number 19. Elemental potassium is a soft silvery-white alkali metal that oxidizes rapidly in air and is very reactive with water, generating sufficient heat to ignite hydrogen emitted in the reaction and burning with a lilac flame. Most industrial chemical applications of potassium employ the relatively high solubility in water of potassium compounds, such as potassium soaps. Potassium metal has only a few special applications, being replaced in most chemical reactions with sodium metal.
Magnesium is a chemical element with symbol Mg and atomic number 12. It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (Group 2, or alkaline earth metals) of the periodic table: they each have the same electron configuration in their outer electron shell producing a similar crystal structure.Magnesium is the ninth most abundant element in the universe.[4][5] It is synthesized in large, aging stars from the sequential addition of three helium nuclei to a carbon nucleus
Calcium is a chemical element with symbol Ca and atomic number 20. Calcium is a soft gray alkaline earth metal, fifth-most-abundant element by mass in the Earth's crust. The ion Ca2+ is also the fifth-most-abundant dissolved ion in seawater by both molarity and mass, after sodium, chloride, magnesium, and sulfate.[3] Free calcium metal is too reactive to occur in nature. Calcium is produced in the explosions at the end of the life of massive starsCalcium is essential for living organisms, in particular in cell physiology, where movement of the calcium ion into and out of the cytoplasm functions as a signal for many cellular processes. As a major material used in mineralization of bone, teethand shells, calcium is the most abundant metal by mass in many animals.
Iron is a chemical element with symbol Fe (from Latin: ferrum) and atomic number 26. It is a metal in the first transition series.[4] It is by mass the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. Like other group 8 elements, iron exists in a wide range of oxidation states, − 2 to +6, although +2 and +3 are the most common. Elemental iron occurs in meteoroids and other low oxygen environments, but is reactive to oxygen and water. Fresh iron surfaces appear lustrous silvery-gray, but oxidize in normal air to give hydrated iron oxides, commonly known as rust. Unlike many other metals which form passivating oxide layers, iron oxides occupy more volume than the metal and thus flake off, exposing fresh surfaces for corrosion.Iron metal has been used since ancient times, although copper alloys, which have lower melting temperatures, were used even earlier in human history.
Copper is a chemical element with symbol Cu (from Latin: cuprum) and atomic number 29. It is a ductile metal with very highthermal and electrical conductivity. Pure copper is soft and malleable; a freshly exposed surface has a reddish-orange color. It is used as a conductor of heat and electricity, a building material, and a constituent of various metal alloys. Copper is essential to all living organisms as a trace dietary mineral because it is a key constituent of the respiratory enzyme complex cytochrome c oxidase. In molluscs and crustacea copper is a constituent of the blood pigment hemocyanin, which is replaced by the iron-complexed hemoglobin in fish and other vertebrates.
Barium is a chemical element with symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallicalkaline earth metal. Because of its high chemical reactivity barium is never found in nature as a free element. Its hydroxide was known in pre-modern history as baryta; this substance does not occur as a mineral, but can be prepared by heating barium carbonate.The most common naturally occurring minerals of barium are barite (barium sulfate, BaSO4) and witherite (barium carbonate, BaCO3), both being insoluble in water.
Silver is a chemical element with symbol Ag (Greek: ά ρ γ υ ρ ο ς á rguros, Latin: argentum, both from the Indo-European root *h₂ erǵ - for " grey" or " shining") and atomic number 47. A soft, white, lustrous transition metal, it possesses the highest electrical conductivity, thermal conductivity and reflectivity of any metal. The metal occurs naturally in its pure, free form (native silver), as an alloy with gold and other metals, and in minerals such as argentite and chlorargyrite. Most silver is produced as a byproduct of copper, gold, lead, and zinc refining.
Silver has long been valued as a precious metal. More abundant than gold, silver metal has in many premodern monetary systems functioned as coinable specie, sometimes even alongside gold
Gold is a chemical element with symbol Au (from Latin: aurum) and atomic number 79. In its purest form, it is a bright, slightly reddish yellow, dense, soft, malleable and ductile metal. Chemically, gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements, and is solid under standard conditions. The metal therefore occurs often in free elemental (native) form, as nuggets or grains, in rocks, in veins and in alluvial deposits. It occurs in a solid solution series with the native element silver (as electrum) and also naturally alloyed with copper and palladium. Less commonly, it occurs in minerals as gold compounds, often with tellurium (gold tellurides).
Zinc, in commerce also spelter, is a chemical element with symbol Zn and atomic number 30. It is the first element of group 12 of the periodic table. In some respects zinc is chemically similar to magnesium: its ion is of similar size and its only common oxidation state is +2. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest mineable amounts are found in Australia, Asia, and the United States. Zinc production includes froth flotation of the ore, roasting, and final extraction using electricity(electrowinning).
Tin is a chemical element with the symbol Sn (for Latin: stannum) and atomic number 50. It is a main group metal in group 14 of the periodic table. Tin shows a chemical similarity to both neighboring group-14 elements, germanium and lead, and has two possible oxidation states, +2 and the slightly more stable +4. Tin is the 49th most abundant element and has, with 10 stable isotopes, the largest number of stable isotopes in the periodic table. It is a silvery, malleable other metal that is not easily oxidized in air, obtained chiefly from the mineral cassiterite where it occurs as tin dioxide, SnO2.
Lead (/lɛ d/) is a chemical element in the carbon group with symbol Pb (from Latin: plumbum) and atomic number 82. Lead is a soft, malleable and heavy post-transition metal. Metallic lead has a bluish-white color after being freshly cut, but it soontarnishes to a dull grayish color when exposed to air. Lead has a shiny chrome-silver luster when it is melted into a liquid. It is also the heaviest non-radioactive element.
Lead is used in building construction, lead-acid batteries, bullets and shot, weights, as part of solders, pewters, fusible alloys, and as a radiation shield.
Mercury is a chemical element with symbol Hg and atomic number 80. It is commonly known as quicksilver and was formerly named hydrargyrum (/haɪ ˈ drɑ rdʒ ə rə m/).[3] A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature.
Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide).
|
|